Abstract
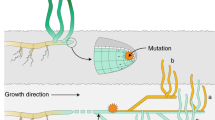
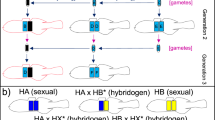
Somatic mutations are an underappreciated source of genetic variation within multi-cellular organisms. The resulting genetic mosaicism should be particularly abundant in large clones of vegetatively propagating angiosperms. Little is known on the abundance and ecological correlates of genetic mosaicism in field populations, despite its potential evolutionary significance. Because sexual reproduction restores genetic homogeneity, we predicted that in facultatively clonally reproducing organisms, the prevalence of genetic mosaicism increases with increasing clonality. This was tested among 33 coastal locations colonized by the ecologically important marine angiosperm Zostera marina, ranging from Portugal to Finland. Genetic mosaics were detectable as complex microsatellite genotypes at two hypervariable loci that revealed additional mosaic alleles, suggesting the presence of one or more divergent cell lineages within the same ramet. The proportions of non-mosaic genotypes in a population sharply decreased below a clonal richness of 0.2. Accordingly, more genetic mosaics were found at the southern and northern limit of the distribution of Z. marina in Europe where sexual reproduction is rare or absent. The genetic mosaics observed at neutral microsatellite markers suggest the possibility of within-clone variation at selectively relevant loci and supports the notion that members of clones are seldom genetically identical.




Similar content being viewed by others
References
Ally D, Ritland K, Otto SP (2008) Can clone size serve as a proxy for clone age? An exploration using microsatellite divergence in Populus tremuloides. Mol Ecol 17:4897–4911
Arnaud-Haond S, Belkhir K (2007) Genclone: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Mol Ecol Notes 7:15–17
Baali-Cherif D, Besnard G (2005) High genetic diversity and clonal growth in relict populations of Olea europaea subsp. laperrinei (Oleaceae) from Hoggar, Algeria. Ann Bot 96:823–830
Bierzychudek P (1985) Patterns in plant parthenogenesis. Experientia 41:1255–1264
Billingham MR, Reusch TBH, Alberto F, Serrão EA (2003) Is asexual reproduction more important at geographical limits? A genetic study of the seagrass Zostera marina in the Ria Formosa, Portugal. Mar Ecol Prog Ser 265:77–83
Buss LW (1983) Somatic variation and evolution. Paleobiology 9:12–16
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Dorken ME, Eckert CG (2001) Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J Ecol 89:339–350
Dorken ME, Neville KJ, Eckert CG (2004) Evolutionary vestigialization of sex in a clonal plant: selection versus neutral mutation in geographically peripheral populations. Proc R Soc Lond Ser B 271:2375–2380
Douhovnikoff V, Dodd RS (2003) Intra-clonal variation and a similarity threshold for identification of clones: application to Salix exigua using AFLP molecular markers. Theor Appl Genet 106:1307–1315
Eckert CG (2001) The loss of sex in clonal plants. Evol Ecol 15:501–520
Fagerström T (1992) The meristem-meristem cycle as a basis for defining fitness in clonal plants. Oikos 63:449–453
Fagerström T, Briscoe DA, Sunnucks P (1998) Evolution of mitotic cell-lineages in multicellular organsims. Trends Evol Ecol 13:117–120
Felsenstein J (1974) The evolutionary advantage of recombination. Genetics 78:737–756
Franks T, Botta R, Thomas MR (2002) Chimerism in grapevines: implication for cultivar identity, ancestry and genetic improvement. Theor Appl Genet 104:192–199
Gill EG, Chao L, Perkins SL, Wolf JB (1995) Genetic mosaicism in plants and clonal animals. Annu Rev Ecol Syst 26:423–444
Honnay O, Bossuyt B (2005) Prolonged clonal growth: escape route or route to extinction? Oikos 108:427–432
Jackson JBC, Buss LW, Cook RE (1985) Population biology and evolution of clonal organisms. Yale University Press, New Haven
Klekowski EJ (1988) Progressive cross- and self-sterility associated with aging in fern clones and perhaps other plants. Heredity 61:247–253
Klekowski EJ (2003) Plant clonality, mutation, diplontic selection and mutational meltdown. Biol J Linnean Soc 79:61–67
Klekowski EJ, Godfrey PJ (1989) Ageing and mutation in plants. Nature 340:389
Les DH, Cleland MA, Waycott M (1997) Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily. System Bot 22:443–463
Loxdale HD, Lushai G (2003) Rapid changes in clonal lines: the death of a ‘sacred cow’. Biol J Linnean Soc 79:3–16
Lushai G, Loxdale HD (2002) The biological improbability of a clone. Genet Res cambridge 79:1–9
McKey D, Elias M, Pujol B, Duputie A (2010) The evolutionary ecology of clonally propagated domesticated plants. New Phytol 186:318–332
Michel A, Arias RS, Scheffler BE, Duke SO, Netherland M, Dayan FE (2004) Somatic-mutation mediated evolution of herbicide resistance in the nonindigenous invasive plant hydrilla (Hydrilla verticillata). Mol Ecol 13:3229–3237
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 1:2–9
Nassar A, Ortiz-Medina E, Leclerc Y, Donnelly D (2008) Periclinal chimeral status of new Brunswick ‘Russet Burbank’ potato. Am J Potato Res 85:432–437
Olsen JL, Stam WT, Coyer JA, Reusch TBH, Billingham M, Boström C, Calvert E, Christie H, Granger S, La Lumiere R, Milchakova N, Oudot Le-Seq M-P, Procaccini G, Sanjabi B, Serrao E, Veldsink J, Widdicombe S, Wyllie-Echeverria (2004) Population differentiation and post-ice age recolonization of the North Atlantic by the seagrass Zostera marina L. Mol Ecol 13:1923–1941
Orive M (2001) Somatic mutations in organisms with complex life histories. Theor Pop Biol 59:235–249
Otto SP, Hastings IM (1998) Mutation and selection within the individual. Genetica 102(103):507–524
Paland S, Lynch M (2006) Transitions to asexuality result in excess amino acid substitutions. Science 311:990–992
Parks JC, Werth CR (1993) A study of spatial features of clones in a population of bracken fern, Pteridium aquilinum (Dennstaedtiaceae). Am J Bot 80:537–544
Pineda-Krch M, Fagerström T (1999) On the potential for evolutionary change in meristemaic cell lineages through intraorganismal selection. J Evol Biol 12:681–688
Pineda-Krch M, Lehtilä K (2002) Cell lineage dynamics in stratified shoot apical mersistems. J Theor Biol 219:495–505
Pineda-Krch M, Lehtilä K (2004) Costs and benefits of genetic heterogeneity within organisms. J Evol Biol 17:1167–1177
Reusch TBH (2000) Five microsatellite loci in eelgrass Zostera marina and a test of cross-species amplification in Z. noltii and Z. japonica. Mol Ecol 9:371–373
Reusch TBH (2002) Microsatellites reveal high population connectivity in eelgrass (Zostera marina) in two contrasting coastal areas. Limnol Oceanogr 47:78–85
Reusch TBH, Boström C, Stam WT, Olsen JL (1999a) An ancient eelgrass clone in the Baltic Sea. Mar Ecol Prog Ser 183:301–304
Reusch TBH, Stam WT, Olsen JL (1999b) Microsatellite loci in eelgrass Zostera marina reveal marked polymorphism within and among populations. Mol Ecol 8:317–322
Reusch TBH, Ehlers A, Hämmerli A, Worm B (2005) Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Natl Acad Sci USA 102:2826–2831
Sakamoto Y, Ishiguro M, Kitagawa G (1986) Akaike information criterion statistics. D. Reidel Publishing Company, Boston
Spielmann D, Brook BW, Frankham R (2004) Most species are not driven to extinction before genetic factors impact them. Proc Natl Acad Sci USA 101:15261–15264
Sutherland WJ, Watkinson AR (1986) Somatic mutation: do plants evolve differently? Nature 320:305
Tomlinson PB (1974) Vegetative morphology and meristem dependence—the foundation of productivity in seagrasses. Aquaculture 4:107–130
Weismann A (1892) Das Keimplasma: eine Theorie der Vererbung. Fischer, Jena
Whitham TG, Slobodchikoff CN (1981) Evolution by individuals, plant herbivore interactions, and mosaics of genetic variability: the adaptive significance of somatic mutations in plants. Oecologia 49:287–292
Acknowledgments
We thank Olivia Roth and Christophe Eizauirre for comments and support with the statistical analysis. Comments by two anonymous referees and the subject matter editor, Isabelle Olivieri, greatly helped improving the manuscript. Silke Carstensen helped with the genotyping. Camilla Roos, Jan Ekebom and Kevin O’Brien are acknowledged for help with field collections in the Archipelago Sea. Finnish Ministry of Environment provided financial support through the VELMU-programme. C.B. was financed by the Academy of Finland, (Project SEASCAPE 200689, grant number 80509).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10682_2010_9436_MOESM1_ESM.eps
Fig. S1 Mean fluorescent signal intensity (± 95% confidence intervals) for main peaks (=alleles, filled) and stutter bands (open) of different heterozygous genotypes for 2 hypervariable microsatellite loci in Zostera marina, ZosmarGA17H (a) and ZosmarGA35 (b), for heterozygous genotypes different one to 3 + repeat units. These confidence intervals were used to distinguish mosaic microsatellite alleles from other artifacts. All diagrams combine at least 3 different independent analyses. The first (longest) allelic peak (Black bar) was set at 1. All eletropherograms were obtained using an ABI 3130xl or 3100 capillary sequencer and the pop4 polymer. Supplementary material 1 (EPS 690 kb)
Rights and permissions
About this article
Cite this article
Reusch, T.B.H., Boström, C. Widespread genetic mosaicism in the marine angiosperm Zostera marina is correlated with clonal reproduction. Evol Ecol 25, 899–913 (2011). https://doi.org/10.1007/s10682-010-9436-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-010-9436-8