Abstract
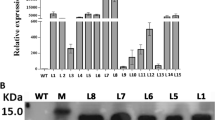
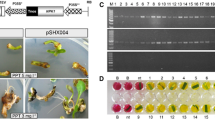
Embryogenic callus of Phalaenopsis amabilis derived from leaf tissue was cocultivated with Agrobacterium tumefaciens strain LBA4404 harboring a plant cloning vector. The vector carried the lipid transfer protein (LTP) encoding gene cloned from cold tolerant Brazilian upland rice cv. IAPAR 9. The highest transformation efficiency (12.16%) was obtained when 1–2 mm calli were infected and cocultivated with 0.4 (OD600) A. tumefaciens for 20 min. Transgene integration of kan-resistant plants was confirmed through polymerase chain reaction analysis and Southern hybridization. Four hundred seventy transgenic plants, each derived from an independent protocorm-like body, were obtained. The expression of rice cold-inducible LTP gene in transgenic P. amabilis improved its adaptive responses to cold stress. The examination of transgenic plants revealed that enhanced cold tolerance was most likely due to the increased accumulation of several compatible solutes such as total soluble sugars, proline, antioxidant superoxide dismutase, decreased accumulation of malondialdehyde, and maintained electrolytes within the membrane compared with controls.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- AS:
-
Acetosyringone
- BAP:
-
6-Benzylaminopurine
- Cef:
-
Cefotaxime
- EDTA:
-
Ethylene diaminetetraacetic acid
- EL:
-
Electrolyte leakage
- Kan:
-
Kanamycin
- LB:
-
Lauria bertani
- LTP:
-
Lipid transfer protein
- MDA:
-
Malondialdehyde
- MET:
-
Methionine
- MS:
-
Murashige and Skoog medium
- NAA:
-
Naphthalene acetic acid
- NBT:
-
Nitroblue tetrazolium
- npt II:
-
Neomycin phosphotransferase II
- PCR:
-
Polymerase chain reaction
- PLB:
-
Protocorm-like body
- pUbi:
-
Plant ubiquitin promoter
- SDW:
-
Sterile distilled water
- SDS:
-
Sodium dodecyl sulfate
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TDZ:
-
Thidiazuron
- TE:
-
Transformation efficiency
- VB6 :
-
Pyridoxine HCl
References
Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH (1987) Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24:324–331
Anchordoguy T, Carpenter JF, Loomis SH, Crowe JH (1988) Mechanisms of interaction of amino acids with phospholipid bilayers during freezing. Biochim Biophys Acta 946:299–306
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem 44:276–287
Belarmino MM, Mii M (2000) Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep 19:435–442
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. Plants J Plant Physiol 160:283–292
Chai ML, Xu CJ, Senthil KK, Kim JY, Kim DH (2002) Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by Agrobacterium tumefaciens. Sci Hortic 96:213–224
Chan YL, Lin KH, Liao LJ, Chen WH, Chan MT (2005) Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res 14:279–288
Choi AM, Lee SB, Cho SH, Inhwan H, Cheol-Goo H, Suh MC (2008) Isolation and characterization of multiple abundant lipid transfer protein isoforms in developing sesame (Sesamum indicum L.) seeds. Plant Physiol Biochem 46:127–139
Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194:541–549
Crowe JH, Crowe LM, Carpenter JF, Rudolph AS, Wistrom CA, Spargo BJ, Anchordoguy TJ (1988) Interactions of sugars with membranes. Biochem Biophy Acta 947:367–384
Eagles CF, Williams J, Louis DV (1993) Recovery after freezing effect of sugar concentration on cold acclimation in Avena sativa L. Lolium perenne L. and L. multiorum Lam. New Phytol 123:477–483
Estruch JJ, Carozzi NB, Desai N, Duck NB, Warren GW, Koziel MG (1997) Transgenic plants: an emerging approach to pest control. Nat Biotechnol 15:137–141
Hughes MA, Dunn MA, Pearce RS, White AJ, Zhang L (1992) An abscisic-acid-responsive, low temperature barley gene has homology with a maize phospholipid transfer protein. Plant Cell Environ 15:861–865
Jung HW, Kim W, Hwang BK (2003) Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell Environ 26:915–928
Kirubakaran SI, Begum SM, Ulaganathan K, Sakthivel N (2008) Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol Biochem 46:918–927
Kurodu H, Sagisaka S, Chiba K (1992) Collapse of peroxide-scavenging systems in apple flower-buds associated with freezing injury. Plant Cell Physiol 33:743–750
Kurokawa T, Itagaki S, Yamaji T, Nakata C, Noda T, Hirano T (2006) Antioxidant activity of a novel extract from bamboo grass (AHSS) against ischemia–reperfusion injury in rat small intestine. Biol Pharm Bull 29:2301–2303
Li CB (2004) Cloning of OsLTP1 gene from the Brazilian upland rice cv. IAPAR 9, its expression analysis and function identification. Dissertation of Institute of Genetics and Developmental Biology, Beijing, Chinese Academy of Sciences
Liau CH, You SJ, Prasad V, Hsiao HH, Lu JC, Yang NS, Chan MT (2003) Agrobacterium tumefaciens-mediated transformation of an Oncidium orchid. Plant Cell Rep 21:993–998
Men S, Ming X, Wang Y, Liu R, Wei C, Li Y (2003) Genetic transformation of two species of orchid by biolistic bombardment. Plant Cell Rep 21:592–598
Mishiba K, Chin DP, Mii M (2005) Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep 24:297–303
Offringa R, Lee F (1995) Isolation and characterization of plant genomic DNA sequences via (inverse) PCR amplification. In: Jones H (ed) Methods in molecular biology: plant gene transfer and expression protocols, vol 49. Humana Press, Totowa, pp 181–195
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pearce RS, Dunn MA, Rixon JE, Harrison P, Hughes MA (1996) Expression of cold-inducible genes and frost hardiness in the crown meristem of young barley (Hordeum vulgare L. cv. Igri) plants grown in different environments. Plant Cell Environ 19:275–290
Polle A (1997) Defense against photooxidative damage in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbour Laboratory Press, Plainview, pp 623–666
Sagisaka S (1985) Injuries of cold acclimatized poplar twigs resulting from enzyme inactivation and substrate depression during frozen storage at ambient temperatures for a long period. Plant Cell Physiol 26:1135–1145
Santarius KA (1992) Freezing of isolated thylakoid membranes in complex media. VIII. Differential cryoprotection by sucrose, proline and glycerol. Physiol Plant 84:87–93
Santoro MM, Liu Y, Khan SMA, Hou L-X, Bolen DW (1992) Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochem 31:5278–5283
SAS Institute Inc (2001) SAS/START user’s guide version 8.2. SAS Institute, Cary
Semiarti E, Indrianto A, Purwantoro A, Isminingsih S, Suseno N, Ishikawa T, Yoshioka Y, Machida Y, Machida C (2007) Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotechnol 24:265–272
Shalaev EY, Steponkus PL (2001) Phase behavior and glass transition of 1, 2-dioleoylphosphaditylethanolamine (DOPE) dehydrated in the presence of sucrose. Biochem Biophys Acta Biomembr 1514:100–116
Shao HB, Liang ZS, Shao MA (2006) Osmotic regulation of 10 wheat (Triticum aestivum L.) genotypes at soil water deficits. Biointer 47:132–139
Shao HB, Chu LY, Lu ZH, Kang CM (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochem 28:1057–1060
Srinivas V, Balasubramanian D (1995) Proline is a protein-compatible hydrotrope. Langmuir 11:2830–2833
Steponkus PL, Uemura M, Webb MS (1993) A contrast of the cryostability of the plasma membrane of winter rye and spring oat-two species that widely differ in their freezing tolerance and plasma membrane lipid composition. In: Steponkus PL (ed) Adv Low-Temp Biol, vol 2. Jai Press, London, pp 211–312
Su V, Hsu BD (2003) Cloning and expression of a putative cytochrome P450 gene that influences the colour of Phalaenopsis flowers. Biotechnol Lett 25:1933–1939
Trunova TI (1982) Mechanisms of winter wheat hardening at low temperature. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress, vol 2. New York, Academic Press, pp 41–47
Walker MA, McKersie BD (1993) Role of ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol 141:234–239
Wise R, Naylor A (1987) Chilling enhanced photooxidation. Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83:278–282
Xuan J, Liu J, Gao H, Hu H, Cheng X (2009) Evaluation of low-temperature tolerance of zoysia grass. Trop Grasslands 43:118–124
Acknowledgments
This work was funded by Chinese National Major Program of Transgenic Research (2009ZX08004-002B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, X., Liu, Y., Mao, S. et al. Genetic transformation of lipid transfer protein encoding gene in Phalaenopsis amabilis to enhance cold resistance. Euphytica 177, 33–43 (2011). https://doi.org/10.1007/s10681-010-0246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0246-4