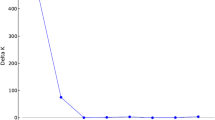
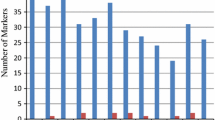
Abstract
Genetic diversity analysis within a species is vital for understanding evolutionary processes at the population and genomic levels. We report a detailed study of molecular diversity, polymorphism and linkage disequilibrium in three groups of rice (Oryza) germplasm accessions based on 176 SSR markers. The first group included 65 rice (O. sativa L.) accessions introduced from seven countries, including five regions of China. The second group included 58 US rice varieties released in the past 25 years. The third group consisted of 54 accessions of rice wild relatives represented by ten different species. The number of alleles per SSR marker ranged from 4 to 32 with a mean of 16 alleles and the polymorphism information content values ranged from 0.43 to 0.91 with a mean of 0.70. The variation in SSR alleles was a significant contribution to the genetic discrimination of the 177 accessions within the three Oryza groups. Analysis of molecular variance identified deviation from Hardy–Weinberg equilibrium. Principal coordinates analysis clearly separated the accessions into their respective three groups. Neighbor-joining phylogenetic cluster reflects the ordination of each accession. Linkage disequilibrium (D′) averaged 0.75 in wild Oryza spp., and about 0.5 in both US and international O. sativa accessions. Our results showed that LD among adjacent loci in both O. sativa and Oryza spp. accessions is strong enough to be detecting marker-trait association via genome-wide scans.





Similar content being viewed by others
References
Agrama HA, Eizenga GC, Yan W (2007) Association mapping of yield and its components in rice cultivars. Mol Breed 19:341–356
Akkaya MS, Bhagwat AA, Cregan PB (1992) Length polymorphisms of simple sequence repeat DNA in soybean. Genetics 132:1131–1139
Bautista NS, Solis R, Kamijima O, Ishii T (2001) RAPD, RFLP and SSLP analyses of phylogenetic relationships between cultivated and wild species of rice. Genes Genet Syst 76:71–79
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314–331
Breseghello F, Sorrells ME (2006) Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172:1165–1177
Brown AHD (1979) Enzyme polymorphism in plant populations. Theor Popul Biol 15:1–42
Buckler ES, Gaut BS, McMullen MD (2006) Molecular and functional diversity of maize. Curr Opin Plant Biol 9:172–176
Chang TT (1995) Rice. In: Smartt J, Simmonds NW (eds) Evolution of crop plants. Longman, UK, pp 147–155
Chen X, Cho YG, McCouch SR (2002) Sequence divergence of rice microsatellites in Oryza and other plant species. Mol Genet Genomics 268:331–343
Cho YG, Ishii T, Temnykh S, Chen X, Lipovich L, McCouch SR, Park WD, Ayres N, Cartinhour S (2000) Diversity of microsatellites derived from genomic libraries and GenBank sequences in rice (Oryza sativa L.). Theor Appl Genet 100:713–722
Eaves IA, Barber RA, Merriman TR (1998) Comparison of linkage disequilibrium in populations from UK and Finland. Am J Hum Gen A221
Eizenga GC, Agrama HA, Lee FN, Yan W, Jia Y (2006) Characterization of newly introduced, Blast resistant rice germplasm. Crop Sci 46:1870–1878
Eizenga GC, Agrama HA, Lee FN (2007) Identifying novel resistance genes in rice wild relatives. In: Norman RJ, Meullenet J-F, Moldenhauer KAK (eds) B.R. Wells Rice Research Studies 2006. Univ Arkansas Agric Exp Stn Res Ser (in press)
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Flint-Garcia SA, Thornsberry JM, Buckler ESD (2003) Structure of linkage disequilibrium in plants. Annu Rev Plant Biol 54:357–357
Garris A, McCouch SR, Kresovich S (2003) Population structure and its effect on haplotype diversity and linkage disequilibrium surrounding the xa5 locus of rice (Oryza sativa L.). Genetics 165:759–769
Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S (2005) Genetic structure and diversity in Oryza sativa L. Genetics 169:1631–1638
Hastings A (1990) In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant Population Genetics, Breeding and Genetic Resources. Sinauer, Sunderland, MA, p 449
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K (2002) A comprehensive review of genetic association studies. Genet Med 4:45–61
Hyten DL, Choi IY, Song Q, Shoemaker RC, Nelson RL, Costa JM, Specht JE, Cregan PB (2007) Highly variable patterns of linkage disequilibrium in multiple soybean populations. Genetics Online:106.06970v1
Ishii T, McCouch SR (2000) Microsatellites and microsynteny in the chloroplast genomes of Oryza and eight other Gramineae species. Theor Appl Genet 100:1257–1266
Ishii T, Xu Y, McCouch SR (2001) Nuclear- and chloroplast- microsatellite variation in A-genome species of rice. Genome 44:658–666
Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290:344–447
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35:25–34
Kraakman ATW, Martínez F, Mussiraliev B, van Eeuwijk FA, Niks RE (2006) Linkage disequilibrium mapping of morphological, resistance, and other agronomically relevant traits in modern spring barley cultivars. Mol Breed 17:41–58
Kraakman ATW, Niks E, van den Berg PM, Stam P, van Eeuwijk FA (2004) Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics 168:435–446
Kraft T, Hansen M, Nilsson N-O (2000) Linkage disequilibrium and fingerprinting in sugar beet. Theor Appl Genet 101:323–326
Kruger SA, Able JA, Chalmers KJ, Langridge P (2004) Linkage disequilibrium analysis of hexaploid wheat. In: Plant & animal genomes XII conf., 10–14 January, San Diego, CA, p 321
Kruglyak L (1999) Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet 22:139–144
Lander ES, Schork NJ (1994) Genetic dissection of complex traits. Science 265:2037–2048
Liu K, Muse S (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Lu H, Redus MA, Coburn JR, Rutger JN, McCouch SR, Tai TH (2005) Population structure and breeding patterns of 145 U.S. rice cultivars based on SSR marker analysis. Crop Sci 45:66–76
Maccaferri M, Sanguinetti MC, Noli E, Tuberosa R (2005) Population structure and long-range linkage disequilibrium in a durum wheat elite collection. Mol Breed 15:271–289
Mather DE, Hyes PM, Chalmers KJ, Eglinton J, Matus I, Richardson K, VonZitzewitz J, Marquez-Cedillo L, Hearnden P, Pal N (2004) Use of SSR marker data to study linkage disequilibrium and population structure in Hordeum vulgare: prospects for association mapping in barley. In: International barley genetics symposium, Brno, Czech Republic, 20–26 June, pp 302–307
Maurer HP, Knaak C, Melchinger AE, Ouzunova M, Frisch M (2006) Linkage disequilibrium between SSR markers in six pools of elite lines of an European breeding program for hybrid maize. Maydica 51:269–280
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Carinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Research 9:199–207
Morrell PL, Toleno DM, Lundy KE, Clegg MT (2005) Low levels of linkage disequilibrium in wild barley (Hordeum vulgare ssp. spontaneum) despite high rates of self-fertilization. Proc Natl Acad Sci USA 102:2442–2447
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc of the National Acad Sci USA 70:3321–3323
Ni J, Colowit PM, Mackill DJ (2002) Evaluation of genetic diversity in rice subspecies using microsatellite markers. Crop Sci 42:601–607
Nordborg M, Borevitz JO, Bergelson J, Berry CC, Chory J, Hagenblad J, Kreitman M, Maloof JN, Noyes T, Oefner PJ, Stahl EA, Weigel D (2002) The extent of linkage disequilibrium in Arabidopsis thaliana. Nat Genet 30:190–193
Nordborg M, Hu TT, Ishino Y, Jhaveri J, Toomanjian C, Zheng H, Bakker E, Calabrese P, Gladstone J, Goyal R, Jakobsson M, Kim S, Morozov Y, Padhukasahasram B, Plagnol V, Rosenberg NA, Shah C, Wall JD, Wang J, Zhao K, Kalbfeisch T, Schulz V, Kreitman M, Bergelson J (2005) The pattern of polymorphism in Arabidopsis thaliana. PLoS Biology 3:1289–1299
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecology Notes 6:288–295
Rakshit S, Rakshit A, Matsumura H, Takahashi Y, Hasegawa Y, Ito A, Ishii T, Miyashita NT, Terauchi R (2007) Large-scale DNA polymorphism study of Oryza sativa and O. rufipogon reveals the origin and divergence of Asian rice. Theor Appl Genet 114:731–743
Remington DL, Thornsberry JM, Matsuola Y, Wilson LM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler IV ES (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci USA 98:11479–11484
Rostoks N, Ramsay L, MacKenzie K, Cardle L, Bhat PR, Roose ML, Svensson JT, Stein N, Varshney RK, Marshall DF, Graner A, Close TJ, Waugh R (2006) Recent history of artificial outcrossing facilitates whole-genome association mapping in elite inbred crop varieties. Proc Natl Acad Sci USA 103:18656–18661
Russell JR, Booth A, Fuller JD, Baum M, Ceccarelli S, Grando S, Powell W (2003) Patterns of polymorphism detected in the chloroplast and nuclear genomes of barley landraces sampled from Syria and Jordan. Theor Appl Genet 107:413–4213
Saitou N (1987) Neighbor-joining method. Mol Biol Evol 4:406–425
Sawyer SL, Mukherjee N, Pakstis AJ, Feuk L, Kidd JR, Brooks AJ, Kidd KK (2005) Linkage disequilibrium patterns vary substantially among populations. Europ J Hum Genet 13:677–686
Schneider S, Excoffier L (1999) Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152:1079–1089
Semon M, Nielsen R, Jones MP, McCouch SR (2005) The population structure of African cultivated rice Oryza glaberrima (Steud.): evidence for elevated levels of LD caused by admixture with O. sativa and ecological adaptation. Genetics 169:1639–1647
Skøt L, Humphreys MO, Armstead I, Heywood S, Skøt KP, Sanderson R, Thomas ID, Chorlton KH, Hamilton NRS (2005) An association mapping approach to identify flowering time genes in natural populations of Lolium perenne (L.). Mol Breed 15:233–245
Stich B, Melchinger AE, Frisch M, Maurer HP, Heckenberger M, Reif JC (2005) Linkage disequilibrium in European elite maize germplasm investigated with SSRs. Theor Appl Genet 111:723–730
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Tenesa A, Knott SA, Ward D, Smith D, Williams JL, Visscher PM (2003) Estimation of linkage disequilibrium in a sample of the United Kingdom dairy cattle population using unphased genotypes. J Anim Sci 81:617–623
Thomson MJ, Septiningsih EM, Suwardjo F, Santoso TJ, Silitonga TS, McCouch SR (2007) Genetic diversity analysis of traditional and improved Indonesian rice (Oryza sativa L.) germplasm using microsatellite markers. Theor Appl Genet 114:731–743
Thornsberry JM, Goodman MM, Doebley J, Kresovich S, Nielsen D, Buckler IV ES (2001) Dwarf8 polymorphisms associate with variation in flowering time. Nat Genet 28:286–289
Weir BS (1996) Genetic data analysis II: materials for discrete population genetic data. Sinauer Associates, Sunderland, MA
Wright S (1978) Evolution and genetics of populations, Vol. IV. The University of Chicago Press, Chicago
Xu Y, Beachell H, McCouch SR (2005) A marker-based approach to broadening the genetic base of rice in the USA. Crop Sci 44:1947–1959
Yu J, Buckler ES (2006) Genetic association mapping and genome organization of maize. Curr Opin Biotech 17:155–160
Acknowledgments
The support of H.A. Agrama and H.R. Refeld from the Arkansas Rice Research and Promotion Board is acknowledged. Contributions of the DB NRRC Genomics Core Facility under the direction of M.H. Jia and technical assistance of Q.P. Ho and H.R. Refeld also are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrama, H.A., Eizenga, G.C. Molecular diversity and genome-wide linkage disequilibrium patterns in a worldwide collection of Oryza sativa and its wild relatives. Euphytica 160, 339–355 (2008). https://doi.org/10.1007/s10681-007-9535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9535-y