Abstract
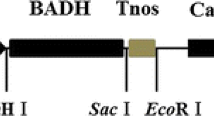
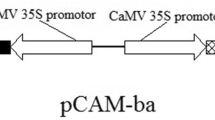
In this research we established a particular vector-free and marker-free plant transformation system of maize to overcome the obstacles of biosafety limits. The BADH gene was introduced into maize by pollen-tube pathway, using the principle of minimum linear length of the transformation element, which was composed of only the BADH gene, expression regulatory sequence (35S CAMV promoter, NOS terminator), and T-DNA border sequence at both sides. Twenty-seven of 2076 transformed samples were positive in PCR amplification and the PCR positive rate of T1 generation was 1.3%. Further Southern blotting results indicated that the BADH gene was integrated into maize genome. Transgenic lines of progeny were examined for tolerance to NaCl by induced salt stress with 250 mM NaCl Hoagland solution. After 15 days of treatment, 73.9–100% of the transgenic seedlings survived and grew well, whereas most wild-type seedlings wilted and showed loss of chlorophyll. Only 8.9% of the wild-type plants survived but gradually died after salt stress. The electrical conductivity of the transgenic line of progeny after salt stress was lower than wild type. The transgenic progeny had higher glycinebetaine and Chlorophyll content than wild type after salt stress.







Similar content being viewed by others
Abbreviations
- BADH:
-
Betaine aldehyde dehydrogenase
- 35S CAMV:
-
35S Cauliflower mosaic virus
- NOS terminator:
-
Nopaline synthase terminator
- CMO:
-
Choline monooxygenase
- HPLC:
-
High performance liquid chromatography
- REC:
-
Relative electrical conductivity
References
Adam AL, Gala AA, Manninger K, Bama B (2000) Inhibition of the development of leaf rust (Puccinia recondita) by treatment of wheat with allopurinol and production of a hypersensitive-like reaction in a compatible host. Plant Pathol 49:317–323
Alia, Hayashi H, Chen THH, Murata N (1998) Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ 21:232–239
Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyIIa and removal of phycocyanin. Biochem Biophys Acta 357:231–245
Artelt P, Grannemann R, Stocking C, Friel J, Bartsch J, Hauser H (1991) The prokaryotic neomycin-resistance encoding gene acts as a transcriptional silencer in eukaryotic cells. Gene 99:249–254
Bajji M, Kinet JM, Lutts S (2001) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation 00:1–10
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Chen SL, Bi WF, Li JK, Wang SS (2000) Quantitative determination of glycinebetaine in plant tissues by reverse phase ion-pair HPLC. Acta Botanica Sinica 42(10):1014–1018
Daniell H (1999) GM crops: Public perception and scientific solutions. Trends Plant Sci 4:467–469
De Vetten N, Wolters A-M, Raemakers K, Van Der Meer I, Ter Stege R, Heeres E, Heeres P, Visser R (2003) A transformation method for obtaining marker-free plants of a cross-pollinating and vegetatively propagated crop. Nat Biotechnol 21:439–442
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fu XD, Duc LT, Fontana S, Bong BB, Tinjuangjun P, Sudhakar D, Twyman RM, Christou P, Kohli A (2000) Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res 9:11–19
Gorham J (1995) Betaines in higher plants: biosynthesis and role in stress metabolism. In: Wallsgrove RM (ed) Amino acids and their derivatives in higher plants. Cambridge University Press, Cambridge, UK, pp 171–203
Guo BH, Zhang YM, Li HJ, Du LQ, Li YX, Zhang JS, Chen SY, Zhu ZQ (2000) Tansformation of wheat with a gene encoding for the betaine aldehyde dehydrogenase (BADH). Acta Bot Sin 42:279–283
Hayashi H, Murata N (1998) Genetically engineered enhancement of salt tolerance in higher plants. In: Satoh K, Murata N (eds) Stress responses of photosynthetic organisms. Elsevier Press, Amsterdam, pp 133–148
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122:747–756
Jia GX, Zhu ZQ, Chang FQ, Li YX (2002) Transformation of tomato with the BADH gene from Atriplex improves salt tolerance. Plant Cell Rep 21:141–146
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854
Leopold AC, Toenniessen RPW (1984) Salinity tolerance in plants. Wiley, New York
Le Rudulier D, Strom AR, Dandekar AM, Smith LT, Valentine RC (1984) Molecular biology of osmoregulation. Science 224:1064–1068
Li QL, Gao XR, Yu XH, Wang XZ, An LJ (2003) Molecular cloing and characterization of betaine aldehyde dehydrogenase gene from Suaeda liaotungensis and its use in improved tolerance to salinity in transgenic tobacco. Biotechnol Lett 25:1431–1436
Li YX, Chang FQ, Du LQ, Guo BH, Li HJ, Zhang JS, Chen SY, Zhu ZQ (2000) Genetic Transformation of watercress with a gene encoding for beatine-aldehyde Dehydrogenase (BADH). Acta Bot Sin 42:480–484
Luo ZX, Wu R (1988) A simple method for the transformation of rice via the pollen-tube pathway. Plant Mol Bio Rep 6(3):165–174
Maas EV, Hoffman GJ (1977) Crop salt tolerance current assessment. J Irrig and Drain Div ASCE 103:115–134
Maas EV, Hoffman GJ, Chaba GD, Poss JA, Shannon MC (1983) Salt sensitivity of corn at various growth stages. Irrig Sci 4:45–57
Matzke MA, Matzke AJM, Eggleston WB (1996) Paramutation and transgene silencing: A common response to invasive DNA? Trends Plant Sci 1:382–388
Moghaieb REA, Saneoka H, Fujita K (2004) Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritime. Plant Sci 166:1345–1349
Muller AE, Kamisugi Y, Gruneberg R, Niedenhof I, Horold RJ, Meyer P (1999) Palindromic sequences and A + T-rich DNA elements promote illegitimate recombination in Nicotiana tabacum. J Mol Biol 291:29–46
Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD (1998) The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J 16:487–496
Otha Y (1986) High-efficiency genetic transformation of maize by a mixture of pollen and exogenous DNA. Proc Natl Acad Sci USA 83:715–719
Puchta H (2000) Removing selectable marker genes: taking the shortcut. Trends Plant Sci 5:273–274
Rathinasabapathi B, McCue KF, Gage DA, Hanson AD (1994) Metabolic engineering of glycine betaine synthesis: plant betaine aldehyde dehydrogenases lacking typical transit peptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance. Planta 193:155–162
Rathinasabapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, Scott P, Golbeck JH, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthesic group characterization and cDNA cloning. Proc Natl Acad Sci USA 94:3454–3458
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Sakamoto A, Murata N (2000) Genetic engineering of glycine betaine synthesis in plants: current status and implication for enhancement of stress tolerance. J Exp Bot 51:81–88
Sakamoto A, Murata N (2001) The use of bacterial choline oxidase, a glycine-betaine-synthesizing enzyme, to create stress-resistant transgenic plants. Plant Physiol 125:180–188
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A Laboratory Mannual. Cold Spring Harbor laboratory, Cold Spring Harbor, New York, USA
Sun Y, Flannigan BA, Setter TL (1999) Regulation of endoreduplication in maize (Zea mays L.) endosperm. Isolation of a novel B1-type cyclin and its quantitative analysis. Plant Mol Biol 41:245–258
Wadsworth GJ, Redinbaugh MG, Scandalios JG (1988) A procedure for the small-scale isolation of plant RNA suitable for RNA blot analysis. Anal Biochem 172:279–283
Wang JX, Sun Y, Cui GM, Hu JJ (2001) Transgenic maize plants obtained by pollen-mediated transformation. Acta Bot Sin 43:275–279
Zeng JZ, Wang DJ, Wu YQ, Zhang J, Zhou WJ, Zhu XP, Xu NZ (1994) Transgenic wheat plants obtained with pollen-tube pathway method. Sci Chin 37:319–325
Zhang YS, Yin XY, Yang AF, Li GS, Zhang JR (2005) Stability of inheritance of transgenes in maize (Zea mays L.) lines produced using different transformation methods. Euphytica 144:11–22
Zhou GY, Weng J (1983) Introduction of exogenous DNA into cotton embryos. Method in Enzymology (101):433–448
Zhou GY, Weng Z, Gong Y, Zeng W (1988) Molecular breeding of agriculture: A technique for introducing exogenous DNA into plants after self-pollination. Sci Agric Sinica 21:1–6
Acknowledgements
The authors are grateful to Prof. Zhang Zhihong, Prof. Wang Shu and Prof. Li Qiuli for technical assistance and valuable discussion. The study was supported by National Agricultural Institute of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Su, Q., Xia, X.Y. et al. The Suaeda liaotungensis kitag betaine aldehyde dehydrogenase gene improves salt tolerance of transgenic maize mediated with minimum linear length of DNA fragment. Euphytica 159, 17–25 (2008). https://doi.org/10.1007/s10681-007-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9451-1