Abstract
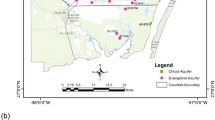
The aim of this paper is to provide a methodology including statistical tools and spatial techniques, in order to identify the various potential sources of chromium (Crtot) in the Sarigkiol basin, Western Macedonia, Greece, where elevated concentrations of Crtot in groundwater have been recorded since 1996. Integrated hydrochemical approach and statistical analyses including Pearson’s correlation coefficient, multivariate statistical analyses (factor analysis and hierarchical cluster analysis), and spatial techniques (Moran’s I spatial autocorrelation index and bivariate local indicator spatial association cluster map) were applied to evaluate the chemical analyses of 73 water samples, from irrigation wells, natural springs, and surface water. Both natural and anthropogenic sources of Crtot were recorded; the first (ultramafic-dominated environment) is strongly depicted on the natural spring water, in which Crtot concentrations as high as ~ 130 μg/L were recorded, whereas the second (agricultural activities) acts synergistically in the irrigation wells of the Sarigkiol basin, in which strong correlations of Crtot, P, and NO3− were defined. The paper highlights its findings by outlining the potential sources of elevated concentrations of Cr6+ in the Sarigkiol basin, stressing the need for a closer attention on the role of agricultural activities as an important, though commonly neglected, anthropogenic source of Crtot in groundwater.
















Similar content being viewed by others
References
Anastopoulos, G. C., & Koukouzas, C. N. (1972). Economic geology of the southern part of Ptolemais lignite basin (Macedonia-Greece). Geology and Geophysics Research, XVI(1), 189 (in Greek).
Amacher, M. C., & Baker, D. E. (1982). Redox reactions involving chromium, plutonium and manganese in soils. Final report DOE/DP/04515-1, Pennsylvania State University, Institution for Research on Land and Water Resources. https://doi.org/10.2172/5030864.
Anselin, L. (1994). Exploratory spatial data analysis and geographic information systems. In New tools for spatial analysis (pp. 45–54). Luxemburg: Eurostat.
Anselin, L. (1995). Local indicators of spatial association—LISA. Geographical Analysis, 27, 93–115. https://doi.org/10.1111/j.1538-4632.1995.tb00338.x.
Anselin, L. (2005). Exploring spatial data with GeoDa™: a workbook. Urbana-Champaign: University of Illinois.
Apollaro, C., Fuoco, I., Brozzo, G., & De Rosa, R. (2019). Release and fate of Cr(VI) in the ophiolitic aquifers of Italy: the role of Fe(III) as a potential oxidant of Cr(III) supported by reaction path modelling. The Science of the Total Environment, 660, 1459–1471. https://doi.org/10.1016/j.scitotenv.2019.01.103.
Armstrong, S., & Soelberg, P. (1968). On the interpretation of factor analysis. November 1968 Psychological Bulletin, 70(5), 361–364. https://doi.org/10.1037/h0026434.
Aschonitis, V. G., Mastrocicco, M., Colombani, N., Salemi, E., Kazakis, N., Voudouris, K., & Castaldelli, G. (2012). Assessment of the intrinsic vulnerability of agricultural land to water and nitrogen losses via deterministic approach and regression analysis. Water, Air, and Soil Pollution, 223, 1605–1614. https://doi.org/10.1007/s11270-011-0968-5.
Ball, J. W., & Izbicki, J. A. (2004). Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Applied Geochemistry, 19, 1123–1135. https://doi.org/10.1016/j.apgeochem.2004.01.011.
Ball, J. W., & McCleskey, R. B. (2003). A new cation-exchange method for accurate field speciation of hexavalent chromium. Talanta, 61, 305–313. https://doi.org/10.1016/S0039-9140(03)00282-0.
Barnes, I., & O’Neil, J. R. (1969). The relationship between fluids in some fresh alpine-type ultramafics and possible modern serpentinization, Western United States. Bulletin of the Geological Society of America, 80, 1947. https://doi.org/10.1130/0016-7606(1969)80[1947:TRBFIS]2.0.CO;2.
Bartlett, M. S. (1950). Tests of significance in factor analysis. British Journal of Statistical Psychology, 3(January), 77–85.
Becquer, T., Quantin, C., Sicot, M., & Boudot, J. P. (2003). Chromium availability in ultramafic soils from New Caledonia. The Science of the Total Environment, 301, 251–261. https://doi.org/10.1016/S0048-9697(02)00298-X.
Bertolo, R., Bourotte, C., Hirata, R., Marcolan, L., & Sracek, O. (2011). Geochemistry of natural chromium occurrence in a sandstone aquifer in Bauru basin. São Paulo State, Brazil Applied Geochemistry, 26, 1353–1363. https://doi.org/10.1016/j.apgeochem.2011.05.009.
Bourotte, C., Bertolo, R., Almodovar, M., & Hirata, R. (2009). Natural occurrence of hexavalent chromium in a sedimentary aquifer in Uraina, state of Sao Paulo, Brazil. Anais da Academia Brasileira de Ciencias, 81, 227–242. https://doi.org/10.1590/S0001-37652009000200009.
Buerge, I. J., & Hug, S. J. (1997). Kinetics and pH dependence of chromium(VI) reduction by iron(II). Environmental Science & Technology, 31, 1426–1432. https://doi.org/10.1021/es960672i.
Byrne, P., Reid, I., & Wood, P. J. (2010). Sediment geochemistry of streams draining abandoned lead/zinc mines in central Wales: the Afon Twymyn. Journal of Soils and Sediments, 10, 683–697.
Camacho, R. J., & Armienta, M. (2000). Natural chromium contamination of groundwater at León Valley, México. Journal of Geochemical Exploration, 68, 167–181. https://doi.org/10.1016/S0375-6742(99)00083-7.
Cheraghi, M., Lorestani, B., & Merrikhpour, H. (2012). Investigation of the effects of phosphate fertilizer application on the heavy metal content in agricultural soils with different cultivation patterns. Biological Trace Element Research, 145, 87–92. https://doi.org/10.1007/s12011-011-9161-3.
Chon, C. M., Kim, J. G., Lee, G. H., & Kim, T. H. (2008). Influence of extractable soil manganese on oxidation capacity of different soils in Korea. Environmental Geology, 55, 763–773. https://doi.org/10.1007/s00254-007-1029-7.
Chowdhury, S. R., & Yanful, E. K. (2010). Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. Journal of Environmental Management, 91, 2238–2247. https://doi.org/10.1016/j.jenvman.2010.06.003.
Cranston, R. E., & Murray, J. W. (1978). The determination of chromium species in natural waters. Analytica Chimica Acta, 99, 275–282. https://doi.org/10.1016/S0003-2670(01)83568-6.
Dagounaki, C., Chrissafis, K., Kassoli-Fournaraki, A., Tsirambides, A., Sikalidis, C., & Paraskevopoulos, K. M. (2004). Thermal characterization of carbonate rocks: Kozani area, North-Western Macedonia, Greece. Journal of Thermal Analysis and Calorimetry, 78, 295–306. https://doi.org/10.1023/B:JTAN.0000042176.86085.44.
Dermatas, D., Mpouras, T., Chrysochoou, M., Panagiotakis, I., Vatseris, C., Linardos, N., Theologou, E., Boboti, N., Xenidis, A., Papassiopi, N., & Sakellariou, L. (2015). Origin and concentration profile of chromium in a Greek aquifer. Journal of Hazardous Materials, 281, 35–46. https://doi.org/10.1016/j.jhazmat.2014.09.050.
Deverel, S. J., & Millard, S. P. (1988). Distribution and mobility of selenium and other trace elements in shallow groundwater of the Western San Joaquin Valley, California. Environmental Science and Technology, 22, 697–702. https://doi.org/10.1021/es00171a013.
Dimitrakopoulos D., Koumantakis J., Poutios G., Heliadis K. (1998). Methods of artificial recharge, in areas with open pit exploitation. Case of South Field open pit, West Macedonia, Greece. Proceedings of 5th international symposium on environmental issues and waste management in energy and mineral production - Swemp ’98, Ankara/Turkey. A.Α. Balkema, Rotterdam/1998; pp. 299–305
Dimitrakopoulos, D., Vasileiou E., Stathopoulos, N., Dimitrakopoulou S. (2016) Estimation of the qualitative characteristics of post mining lakes in different lignite fields in Greece. In: Drebenstedt, C. & Paul, M.: IMWA 2016—mining meets water—conflicts and solutions. pp. 219–226; Freiberg/Germany (TU Bergakademie Freiberg)
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environmental Science & Technology, 37, 4182–4189. https://doi.org/10.1021/es030309t.
Dunteman, G. H. (1989). Principal components analysis. Quantitative applications in the social sciences: Sage.
Eary, L. E., & Rai, D. (1987). Kinetics of chromium(III) oxidation to chromium(VI) by reaction with manganese dioxide. Environmental Science & Technology, 21, 1187–1193. https://doi.org/10.1021/es00165a005.
Economou-Eliopoulos, M., Megremi, I., Atsarou, C., Theodoratou, C., & Vasilatos, C. (2013). (2013). Spatial evolution of the chromium contamination in soils from the Assopos to Thiva basin and C. Evia (Greece) and potential source(s): anthropogenic versus natural processes. Geosciences. https://doi.org/10.3390/geosciences3020140.
Emsley, J. (2001). Nature’s building blocks: an A-Z guide to the elements. Oxford University Press. pp. 240–242. ISBN 0-19-850341-5
Engström, E., Mörtberg, U., Karlström, A., & Mangold, M. (2017). Applying spatial regression to evaluate risk factors for microbiological contamination of urban groundwater sources in Juba, South Sudan. Hydrogeology Journal, 25, 1077–1091. https://doi.org/10.1007/s10040-016-1504-x.
Evans, J. D. (1996). Straightforward statistics for the behavioral sciences. Belmont: Thomson Brooks/Cole Publishing Co..
Fantoni, D., Brozzo, G., Canepa, M., Cipolli, F., Marini, L., Ottonello, G., & Vetuschi Zuccolini, M. (2002). Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environmental Geology, 42, 871–882. https://doi.org/10.1007/s00254-002-0605-0.
Farnham, I. M., Singh, A. K., Stetzenbach, K. J., & Johannesson, K. H. (2002). Treatment of nondetects in multivariate analysis of groundwater geochemistry data. In: Chemometrics and intelligent laboratory systems. https://doi.org/10.1016/S0169-7439(01)00201-5
Fendorf, S. E., Fendorf, M., Sparks, D. L., & Gronsky, R. (1992). Inhibitory mechanisms of Cr(III) oxidation by δ-MnO2. Journal of Colloid and Interface Science, 153, 37–54. https://doi.org/10.1016/0021-9797(92)90296-X.
Frisbie, M. D., Phillips, F. M., Weissmann, G. S., Brooks, P. D., Wilson, J. L., Campbell, A. R., & Liu, F. (2012). Unraveling the mysteries of the large watershed black box: implications for the streamflow response to climate and landscape perturbations. Geophysical Research Letters, 39. https://doi.org/10.1029/2011GL050416.
Fytianos, K., Tsaniklidi, B., & Voudrias, E. (1998). Leachability of heavy metals in Greek fly ash from coal combustion. Environment International, 24, 477–486. https://doi.org/10.1016/S0160-4120(98)00027-0.
Gao, Y., & Mucci, A. (2001, 2001). Acid base reaction, phosphate and arsenate complexation, and their competitive adsorption at the surface of goethite in 0.7 M NaCl solution. Geochimica et Cosmochimica Acta. https://doi.org/10.1016/S0016-7037(01)00589-0.
Georgakopoulos, A. (2003). Chemistry and morphology of fly ash samples from the main lignite power stations of Northern Greece. Proceedings of 8th international conference on environmental science and technology, pp. 256–263.
Georgakopoulos, A., Filippidis, A., Kassoli-Fournaraki, A., Iordanidis, A., Fernández-Turiel, J. L., Llorens, J. F., & Gimeno, D. (2002). Environmentally important elements in fly ashes and their leachates of the power stations of Greece. Energy Sources, 24, 83–91. https://doi.org/10.1080/00908310252712325.
Georgopoulos, G., Mitsis, I., Argyraki, A., & Stamatakis, M. (2018). Environmental availability of ultramafic rock derived trace elements in the fumarolic - geothermal field of Soussaki area, Greece. Applied Geochemistry, 92, 9–18. https://doi.org/10.1016/j.apgeochem.2018.02.010.
Gonzalez, A. R., Ndung’u, K., & Flegal, A. R. (2005). Natural occurrence of hexavalent chromium in the Aromas Red Sands Aquifer, California. Environmental Science and Technology, 39, 5505–5511. https://doi.org/10.1021/es048835n.
Gorgij, A. D., Kisi, O., Moghaddam, A. A., & Taghipour, A. (2017). Groundwater quality ranking for drinking purposes, using the entropy method and the spatial autocorrelation index. Environment and Earth Science, 76. https://doi.org/10.1007/s12665-017-6589-6.
Grekousis, G., & Gialis, S. (2018). More flexible yet less developed? Spatio-temporal analysis of labor flexibilization and gross domestic product in crisis-hit European Union regions. Social Indicators Research, 143, 505–524. https://doi.org/10.1007/s11205-018-1994-0.
Grigorakou, E., Dimitrakopoulos, D., & Koumantakis, J. (2002). Sensitivity analysis of parameters affecting the dewatering process of an open pit: case-study of South Field, Greece. Acta Universitatis Carolinae. Geologica., 46(2–3), 665–669.
Gülaçar, O. F., & Delaloye, M. (1976). Geochemistry of nickel, cobalt and copper in alpine-type ultramafic rocks. Chemical Geology, 17, 269–280. https://doi.org/10.1016/0009-2541(76)90041-3.
Gunaalan, K., Ranagalage, M., Gunarathna, M., Kumari, M., Vithanage, M., Srivaratharasan, T., Saravanan, S., & Warnasuriya, T. W. S. (2018). Application of geospatial techniques for groundwater quality and availability assessment: a case study in Jaffna Peninsula, Sri Lanka. ISPRS International Journal of Geo-Information, 7. https://doi.org/10.3390/ijgi7010020.
Gunier, R. B., Ward, M. H., Airola, M., Bell, E. M., Colt, J., Nishioka, M., Buffler, P. A., Reynolds, P., Rull, R. P., Hertz, A., Metayer, C., & Nuckols, J. R. (2011). Determinants of agricultural pesticide concentrations in carpet dust. Environmental Health Perspectives, 119, 970–976. https://doi.org/10.1289/ehp.1002532.
Guo, H., Wen, D., Liu, Z., Jia, Y., & Guo, Q. (2014). A review of high arsenic groundwater in Mainland and Taiwan, China: distribution, characteristics and geochemical processes. Applied Geochemistry, 41, 196–217. https://doi.org/10.1016/j.apgeochem.2013.12.016.
Halim, M. A., Majumder, R. K., Nessa, S. A., Oda, K., Hiroshiro, Y., Saha, B. B., Hassain, S. M., Latif, S. A., Islam, M. A., & Jinno, K. (2009). Groundwater contamination with arsenic in Sherajdikhan, Bangladesh: geochemical and hydrological implications. Environmental Geology, 58, 73–84. https://doi.org/10.1007/s00254-008-1493-8.
Hausladen, D. M., Alexander-Ozinskas, A., McClain, C., & Fendorf, S. (2018). Hexavalent chromium sources and distribution in California groundwater. Environmental Science & Technology, 52, 8242–8251. https://doi.org/10.1021/acs.est.7b06627.
Hem, J.D. (1985). Study and Interpretation of the Chemical Characteristics of Natural Water. 3rd Edition, US Geological Survey Water-Supply Paper 2254, University of Virginia, Charlottesville, 263 p
Hermann, R., & Neumann-Mahlkau, P. (1985). The mobility of zinc, cadmium, copper, lead, iron and arsenic in ground water as a function of redox potential and pH. The Science of the Total Environment, 43, 1–12. https://doi.org/10.1016/0048-9697(85)90027-0.
Hjelmar, O. (1990). Leachate from land disposal of coal fly ash. Waste Management & Research, 8, 429–449. https://doi.org/10.1177/0734242X9000800170.
Islam, A. R. M. T., Ahmed, N., Bodrud-Doza, M., & Chu, R. (2017). Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environmental Science and Pollution Research, 24, 26350–26374. https://doi.org/10.1007/s11356-017-0254-1.
Izbicki, J. A., Wright, M. T., Seymour, W. A., McCleskey, R. B., Fram, M. S., Belitz, K., & Esser, B. K. (2015). Cr(VI) occurrence and geochemistry in water from public-supply wells in California. Applied Geochemistry, 63, 203–217. https://doi.org/10.1016/j.apgeochem.2015.08.007.
Izquierdo, M., & Querol, X. (2012). Leaching behaviour of elements from coal combustion fly ash: an overview. International Journal of Coal Geology, 94, 54–66. https://doi.org/10.1016/j.coal.2011.10.006.
Jacobs, J., & Testa, S. M. (2004). Overview of chromium(VI) in the environment: background and history. In Chromium handbook (pp. 1–21). Boca Raton: CRC.
Jahangir, M. M. R., Johnston, P., Khalil, M. I., & Richards, K. G. (2012). Linking hydrogeochemistry to nitrate abundance in groundwater in agricultural settings in Ireland. Journal of Hydrology, 448-449, 212–222. https://doi.org/10.1016/j.jhydrol.2012.04.054.
Jha, M. K., Chowdhury, A., Chowdary, V. M., & Peiffer, S. (2007). Groundwater management and development by integrated remote sensing and geographic information systems: prospects and constraints. Water Resources Management, 21, 427–467. https://doi.org/10.1007/s11269-006-9024-4.
Johnson, D. S., Conn, P. B., Hooten, M. B., Ray, J. C., & Pond, B. A. (2013). Spatial occupancy models for large data sets. Ecology., 94, 801–808. https://doi.org/10.1890/12-0564.1.
Johnson, C. A., & Xyla, A. G. (1991). The oxidation of chromium(II1) to chromium(VI) on the surface of manganate (y-MnOOH). Geochimica et Cosmochimimica Acta., 55, 2861–2866. https://doi.org/10.1016/0016-7037(91)90451-A.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika., 23, 187–200. https://doi.org/10.1007/BF02289233.
Kaiser, H.-F. (1970). A second generation little jiffy. Psychometrika, 35(4), 401–415.
Kantiranis, N., Georgakopoulos, A., Filippidis, A., & Drakoulis, A. (2004). Mineralogy and organic matter content of bottom ash samples from Agios Dimitrios power plant, Greece. Bulletin of the Geological Society of Greece, 36, 320–326. https://doi.org/10.12681/bgsg.16673.
Kaprara, E., Kazakis, N., Simeonidis, K., Coles, S., Zouboulis, A. I., Samaras, P., & Mitrakas, M. (2015). Occurrence of Cr(VI) in drinking water of Greece and relation to the geological background. Journal of Hazardous Materials, 281, 2–11. https://doi.org/10.1016/j.jhazmat.2014.06.084.
Kazakis, N., Kantiranis, N., Kalaitzidou, K., Kaprara, M., Mitrakas, M., Frei, R., et al. (2017). Origin of hexavalent chromium in groundwater: the example of Sarigkiol basin, Northern Greece. Science of the Total Environment, 593-594, 552–566. https://doi.org/10.1016/j.scitotenv.2017.03.128.
Kazakis, N., Kantiranis, N., Voudouris, K. S., Mitrakas, M., Kaprara, E., & Pavlou, A. (2015). Geogenic Cr oxidation on the surface of mafic minerals and the hydrogeological conditions influencing hexavalent chromium concentrations in groundwater. The Science of the Total Environment, 514, 224–238. https://doi.org/10.1016/j.scitotenv.2015.01.080.
Kazakis, N., Kantiranis, N., Kalaitzidou, K., Kaprara, E., Mitrakas, M., Frei, R., Vargemezis, G., Vogiatzis, D., Zouboulis, A., & Filippidis, A.(2018) Environmentally available hexavalent chromium in soils and sediments impacted by dispersed fly ash in Sarigkiol basin (Northern Greece). Environmental Pollution 235:632–641. https://doi.org/10.1016/j.envpol.2017.12.117.
Kelepertzis, E., Argyraki, A., & Daftsis, E. (2012). Geochemical signature of surface water and stream sediments of a mineralized drainage basin at NE Chalkidiki, Greece: a pre-mining survey. Journal of Geochemical Exploration, 114, 70–81. https://doi.org/10.1016/j.gexplo.2011.12.006.
Koilakos, D. I. (2017). Aspects of hexavalent chromium pollution of Thebes Plain aquifer, Boeotia. Greece. Water (Switzerland)., 9. https://doi.org/10.3390/w9080611.
Kotaś, J., & Stasicka, Z. (2000). Chromium occurrence in the environment and methods of its speciation. Environmental Pollution, 107, 263–283. https://doi.org/10.1016/S0269-7491(99)00168-2.
Koumantakis I., (1999). ELIMEIA—development of methodology for water resources management and artificial recharge in regions of lignite exploitation. The case study of South Lignite Field, of Ptolemais basin. Final report, NTUA.
Kožuh, N., Štupar, J., & Gorenc, B. (2000). Reduction and Oxidation Processes of Chromium in Soils. Environmental Science & Technology, 34(1):112–119
Kratz, S., Schick, J., & Schnug, E. (2016). Trace elements in rock phosphates and P containing mineral and organo-mineral fertilizers sold in Germany. The Science of the Total Environment, 542, 1013–1019. https://doi.org/10.1016/j.scitotenv.2015.08.046.
Krüger, O., Fiedler, F., Adam, C., Vogel, C., & Senz, R. (2017). Determination of chromium (VI) in primary and secondary fertilizer and their respective precursors. Chemosphere., 182, 48–53. https://doi.org/10.1016/j.chemosphere.2017.05.011.
Lilli, M. A., Nikolaidis, N. P., Karatzas, G. P., & Kalogerakis, N. (2019). Identifying the controlling mechanism of geogenic origin chromium release in soils. Journal of Hazardous Materials, 366, 169–176. https://doi.org/10.1016/j.jhazmat.2018.11.090.
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. The Science of the Total Environment, 313, 77–89. https://doi.org/10.1016/S0048-9697(02)00683-6.
Louloudis, G. (1991). Hydrogeological conditions of South lignite bearing field of Ptolemais region. Confrontation of groundwater problems during the exploitation. Ph.D. thesis. National Technical University of Athens.
Machiwal, D., & Jha, M. K. (2015). Identifying sources of groundwater contamination in a hard-rock aquifer system using multivariate statistical analyses and GIS-based geostatistical modeling techniques. Journal of Hydrology: Regional Studies. doi: https://doi.org/10.1016/j.ejrh.2014.11.005
Margiotta, S., Mongelli, G., Summa, V., Paternoster, M., & Fiore, S. (2012). Trace element distribution and Cr(VI) speciation in Ca-HCO3 and Mg-HCO3 spring waters from the northern sector of the Pollino massif, Southern Italy. Journal of Geochemical Exploration, 115, 1–12. https://doi.org/10.1016/j.gexplo.2012.01.006.
Marques, J. M., Carreira, P. M., Carvalho, M. R., Matias, M. J., Goff, F. E., Basto, M. J., Graça, R. C., Aires-Barros, L., & Rocha, L. (2008). Origins of high pH mineral waters from ultramafic rocks, Central Portugal. Applied Geochemistry, 23, 3278–3289. https://doi.org/10.1016/j.apgeochem.2008.06.029.
Mason, B., & Moore, C. (1982). Principles of geochemistry. New York: Wiley.
Matong, J. M., Nyaba, L., & Nomngongo, P. N. (2017). Determination of As, Cr, Mo, Sb, Se and V in agricultural soil samples by inductively coupled plasma optical emission spectrometry after simple and rapid solvent extraction using choline chloride-oxalic acid deep eutectic solvent. Ecotoxicology and Environmental Safety, 135, 152–157. https://doi.org/10.1016/j.ecoenv.2016.09.033.
McLay, C. D. A., Dragten, R., Sparling, G., & Selvarajah, N. (2001). Predicting groundwater nitrate concentrations in a region of mixed agricultural land use: a comparison of three approaches. Environmental Pollution, 115, 191–204. https://doi.org/10.1016/S0269-7491(01)00111-7.
Megremi, I., Vasilatos, C., Vassilakis, E., & Economou-Eliopoulos, M. (2019). Spatial diversity of Cr distribution in soil and groundwater sites in relation with land use management in a Mediterranean region: the case of C. In Evia and Assopos-Thiva basins. Greece. Science of the: Total Environment. https://doi.org/10.1016/j.scitotenv.2018.09.186.
Meinikmann, K., Lewandowski, J., & Hupfer, M. (2015). Phosphorus in groundwater discharge—a potential source for lake eutrophication. Journal of Hydrology, 524, 214–226. https://doi.org/10.1016/j.jhydrol.2015.02.031.
Mills, C. T., Morrison, J. M., Goldhaber, M. B., & Ellefsen, K. J. (2011). Chromium(VI) generation in vadose zone soils and alluvial sediments of the southwestern Sacramento Valley, California: a potential source of geogenic Cr(VI) to groundwater. Applied Geochemistry, 26, 1488–1501. https://doi.org/10.1016/j.apgeochem.2011.05.023.
Modaihsh, A., Al-Swailem, M., & Mahjoub, M. (2004). Heavy metals content of commercial inorganic fertilizers used in the Kingdom of Saudi Arabia. Agricultural and Marine Sciences, 9, 21–25.
Molina, M., Aburto, F., Calderón, R., Cazanga, M., & Escudey, M. (2009). Trace element composition of selected fertilizers used in Chile: phosphorus fertilizers as a source of long-term soil contamination. Soil and Sediment Contamination, 18, 497–511. https://doi.org/10.1080/15320380902962320.
Moran, P. A. P. (1948). The interpretation of statistical maps. Journal of the Royal Statistical Society: Series B: Methodological, 10, 243–251. https://doi.org/10.1111/j.2517-6161.1948.tb00012.x.
Morrison, J. M., Goldhaber, M. B., Lee, L., Holloway, J. A. M., Wanty, R. B., Wolf, R. E., & Ranville, J. F. (2009). A regional-scale study of chromium and nickel in soils of Northern California, USA. Applied Geochemistry, 24, 1500–1511. https://doi.org/10.1016/j.apgeochem.2009.04.027.
Mortvedt, J. J. (1996). Heavy metal contaminants in inorganic and organic fertilizers. Fertilizer Research, 43, 55–61.
Nacke, H., Gonçalves, A. C., Schwantes, D., Nava, I. A., Strey, L., & Coelho, G. F. (2013). Availability of heavy metals (Cd, Pb, and Cr) in agriculture from commercial fertilizers. Archives of Environmental Contamination and Toxicology, 64, 537–544. https://doi.org/10.1007/s00244-012-9867-z.
Nanos, N., Grigoratos, T., Rodríguez Martín, J. A., & Samara, C. (2015). Scale-dependent correlations between soil heavy metals and As around four coal-fired power plants of Northern Greece. Stochastic Environmental Research and Risk Assessment, 29(6), 1531–1543.
Neal, C., & Stanger, G. (1983). Hydrogen generation from mantle source rocks in Oman. Earth and Planetary Science Letters, 66, 315–320. https://doi.org/10.1016/0012-821X(83)90144-9.
Nnorom I.C., Ewuzieewuzieug U., Eze S.O. (2019). Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. 24 Jan 2019. Volume 5, Issue 1. Hydrology, Geochemistry, Geology, Natural Hazards, Environmental Science. https://doi.org/10.1016/j.heliyon.2019.e01123
Nriagu, J. O., & Nieboer, E. (1988). Chromium in the natural and human environments. Advances in Environmental Science and Technology. John Wiley and Sons. New York, 20, 1–501.
Nuamah, D. O. B. (2016). Heavy metal source identification and analysis using multivariate statistical methods in soils from Akuse area, South Easter Ghana. Geosciences and Engineering, 5(8), 124–134.
Nziguheba, G., & Smolders, E. (2008). Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. The Science of the Total Environment, 390, 53–57. https://doi.org/10.1016/j.scitotenv.2007.09.031.
Oze, C. (2003). Chromium geochemistry of serpentines and serpentinites and serpentine soils. Ph.D thesis. Stanford University.
Oze, C., Bird, D. K., & Fendorf, S. (2007). Genesis of hexavalent chromium from natural sources in soil and groundwater. Proceedings of the National Academy of Sciences, 104, 6544–6549. https://doi.org/10.1073/pnas.0701085104.
Oze, C., Fendorf, S., Bird, D. K., & Coleman, R. G. (2004). Chromium geochemistry of serpentine soils. International Geology Review, 46, 97–126. https://doi.org/10.2747/0020-6814.46.2.97.
Papadopoulos, K., & Lappas, I. (2014). Groundwater quality degradation due to Cr6+ presence in Schinos area, prefecture of Corinth, Central Greece. 10th International Hydrogeological Congress of Greece, Thessaloniki.
Papazotos, P., Vasileiou, E., & Perraki, M. (2019). The synergistic role of agricultural activities in groundwater quality in ultramafic environments: the case of the Psachna basin, Central Euboea. Greece. Environmental Monitoring and Assessment, 191, 317. https://doi.org/10.1007/s10661-019-7430-3.
Perraki M. (2016). Mineralogical, petrological and geochemical study of heavy minerals with emphasis on chromium in the geological formations (ultrabasic rocks, lignite, clay formations) and the coal-fired products (fly ash) and the quality of surficial and underground aquifers of the Sarigkiol basin (NW Greece). NTUA, final report, p. 826.
Petaloti, C., Kouras, A., Kouimtzis, T. (2003). Heavy metals and other elements distribution in the soil of Eordea basin, W. Macedonia, Greece. Proceedings of the 8th International Conference on Environmental Science and Technology, Lemnos Island, Greece, 8–10 September 2003. Full paper Vol. A, pp. 705–712.
Petrotou, A., Skordas, K., Papastergios, G., & Filippidis, A. (2012). Factors affecting the distribution of potentially toxic elements in surface soils around an industrialized area of northwestern Greece. Environment and Earth Science, 65, 823–833. https://doi.org/10.1007/s12665-011-1127-4.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Eos, Transactions of the American Geophysical Union, 25, 914. https://doi.org/10.1029/TR025i006p00914.
Pourbaix, M. (1966). Atlas of electrochemical equilibria in aqueous solutions (English ed.). Oxford: Pergamon.
Pyrgaki, K., Argyraki, A., Kelepertzis, E., Paraskevopoulou, V., Botsou, F., Dassenakis, E., Mitsis, I., & Skourtsos, E. (2016). Occurrence of hexavalent chromium in the ophiolite related aquifers of Loutraki and Schinos areas. Bulletin of the Geological Society of Greece, 50, 2261–2270. https://doi.org/10.12681/bgsg.14292.
Rai, D., Eary, L. E., & Zachara, J. M. (1989). Environmental chemistry of chromium. The Science of the Total Environment, 86, 15–23. https://doi.org/10.1016/0048-9697(89)90189-7.
Rai, D., Sass, B. M., & Moore, D. A. (1987). Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorganic Chemistry, 26, 345–349. https://doi.org/10.1021/ic00250a002.
Rakhunde, R., Deshpande, L., & Juneja, H. D. (2012). Chemical speciation of chromium in water: a review. Critical Reviews in Environmental Science and Technology, 42, 776–810. https://doi.org/10.1080/10643389.2010.534029
Rao, N. R., & Prasad, P. R. (1997). Phosphate pollution in the groundwater of lower Vamsadhara River basin, India. Environmental Geology, 31, 117–122.
Reddy, K. J., Wang, L., & Gloss, S. P. (1995). Solubility and mobility of copper, zinc and lead in acidic environments. Plant and Soil, 171, 53–58. https://doi.org/10.1007/BF00009564.
Reimann C. & Carirat, P. (1998). Chemical elements in the environment. Factsheets for the geochemist and environmental scientist. ix+398. pp. Berlin: Springer.
Remoundaki, E., Vasileiou, E., Philippou, A., Perraki, M., Kousi, P., Hatzikioseyian, A., & Stamatis, G. (2016). Groundwater deterioration: the simultaneous effects of intense agricultural activity and heavy metals in soil. Procedia Engineering., 162, 545–552. https://doi.org/10.1016/j.proeng.2016.11.099.
Richard, F. C., & Bourg, A. C. M. (1991). Aqueous geochemistry of chromium: a review. Water Research, 25, 807–816. https://doi.org/10.1016/0043-1354(91)90160-R.
Robertson, F.N. (1991). Geochemistry of ground water in alluvial basins of Arizona and adjacent parts of Nevada, New Mexico, and California. US Geological Survey Professional Paper 1406-C, 90 p. http://pubs.er.usgs.gov/publication/pp1406. Accessed 10 Apr 2019
Robertson, F. N. (1989). Arsenic in ground-water under oxidizing conditions, south-west United States. Environmental Geochemistry and Health, 11, 171–185.
Robertson, F. N. (1975). Hexavalent chromium in the ground water in Paradise Valley, Arizona. Groundwater, 13, 516–527. https://doi.org/10.1111/j.1745-6584.1975.tb03621.x.
Sharma, S.K., Petrusevski, B., & Amy, G. (2008). Chromium removal from water: a review. Journal of Water Supply: Research and Technology-Aqua, 57(8):541–553. https://doi.org/10.2166/aqua.2008.080.
Shomar, B. H., Müller, G., & Yahya, A. (2005). Geochemical features of topsoils in the Gaza Strip: natural occurrence and anthropogenic inputs. Environmental Research, 98, 372–382. https://doi.org/10.1016/j.envres.2004.10.008.
Singh, H., Singh, D., Singh, S.K., & Shukla, D.N. (2017). Assessment of river water quality and ecological diversity through multivariate statistical techniques, and earth observation dataset of rivers Ghaghara and Gandak, India. International Journal of River Basin Management, 15(3):347–360. https://doi.org/10.1080/15715124.2017.1300159.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568. https://doi.org/10.1016/S0883-2927(02)00018-5.
Smedley, P. L., & Kinniburgh, D. G. (2017). Molybdenum in natural waters: a review of occurrence, distributions and controls. Applied Geochemistry, 84, 387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008.
Spadoni, M., Voltaggio, M., Sacchi, E., Sanam, R., Pujari, P. R., Padmakar, C., Labhasetwar, P. K., & Wate, S. R. (2014). Impact of the disposal and re-use of fly ash on water quality: the case of the Koradi and Khaperkheda thermal power plants (Maharashtra, India). The Science of the Total Environment, 479-480, 159–170. https://doi.org/10.1016/j.scitotenv.2014.01.111.
Sperling, M., Xu, S., & Welz, B. (1992). Determination of chromium(III) and chromium(VI) in water using flow injection on-line preconcentration with selective adsorption on activated alumina and flame atomic absorption spectrometric detection. Analytical Chemistry, 64, 3101–3108. https://doi.org/10.1021/ac00048a007.
Stamatis, G., Alexakis, D., Gamvroula, D., & Migiros, G. (2011). Groundwater quality assessment in Oropos-Kalamos basin, Attica, Greece. Environmental Earth Sciences, 64, 973–988. https://doi.org/10.1007/s12665-011-0914-2.
Steube, C., Richter, S., & Griebler, C. (2009). First attempts towards an integrative concept for the ecological assessment of groundwater ecosystems. Hydrogeology Journal, 17, 23–35. https://doi.org/10.1007/s10040-008-0346-6.
Tashakor, M., Modabberi, S., van der Ent, A., & Echevarria, G. (2018). Impacts of ultramafic outcrops in Peninsular Malaysia and Sabah on soil and water quality. Environmental Monitoring and Assessment, 190, 333. https://doi.org/10.1007/s10661-018-6668-5.
Tziritis, E., Kelepertzis, E., Korres, G., Perivolaris, D., & Repani, S. (2012). Hexavalent chromium contamination in groundwaters of Thiva basin, Central Greece. Bulletin of Environmental Contamination and Toxicology, 89, 1073–1077. https://doi.org/10.1007/s00128-012-0831-4.
USEPA (2014). Toxic and priority pollutants under the clean water act. Available at: https://www.epa.gov/eg/toxicandpriority-pollutants-under-clean-water-act#priority. Accessed 15 Apr 2019
UjevićBošnjak, M., Capak, K., Jazbec, A., Casiot, C., Sipos, L., Poljak, V., & Dadić, Ž. (2012). Hydrochemical characterization of arsenic contaminated alluvial aquifers in Eastern Croatia using multivariate statistical techniques and arsenic risk assessment. Science of the Total Environment, 420, 100–110.
Vanek, V. (1991). Riparian zone as a source of phosphorus for a groundwater-dominated lake. Water Research, 25(4), 409–418.
Vasilatos, C., Megremi, I., Economou-Eliopoulos, M., & Mitsis, I. (2008). Hexavalent chromium and other toxic elements in natural waters in the Thiva–Tanagra–Malakasa basin, Greece. Hellenic Journal of Geosciences, 43, 57–66.
Vasileiou, E., Perraki M., & Dimitrakopoulos D. (2015). Using leaching tests to investigate mine water contamination. The case study of Open South Lignite Field, Western Macedonia, Greece. Volume 40, 2015, Freiberg Online Geoscience (FOG) Electronic Journal Registered under ISSN 1434-7512
Vasileiou, E., Dimitrakopoulos, D., Papazotos, P., Oikonomopoulos, I., Stathopoulos, N., Skliros, V., & Perraki, M. (2018). Do lignite combustion products affect the groundwater quality near power plants of the Western Macedonia Lignite Center? 14th international symposium of continuous surface mining, Thessaloniki, Greece, September 23–26, 2018
Vasileiou, E., Perraki, M., & Dimitrakopoulos, D. (2017). Hydrochemical characteristics of mine water in the lignite South Field, Western Macedonia, Greece. Proceedings of 11th hydrogeological congress, 4–7 October, Athens
Vasileiou, E., Perraki, M., Stamatis, G., & Gartzos, E. (2014). The effects of water rock interaction and the human activities on the occurrence of hexavalent chromium in waters. The case study of the Psachna basin, Central Euboea, Greece. EGU General Assembly 2014, 27 April–2 May, 2014 in Vienna, Austria, id.15467
Vengosh, A., Coyte, R., Karr, J., Harkness, J. S., Kondash, A. J., Ruhl, L. S., Merola, R. B., & Dywer, G. S. (2016). Origin of hexavalent chromium in drinking water wells from the Piedmont aquifers of North Carolina. Environmental Science & Technology Letters, 3, 409–414. https://doi.org/10.1021/acs.estlett.6b00342.
Voudouris, K. (2009). Assessing groundwater pollution risk in Sarigkiol basin, NW Greece. In: River pollution research progress, Chapter 7, 265–281. Nova Science Publishers Inc. (Eds: M. Gallo and M. Herrari). ISBN 978-1-60456-643-7.
Voutsis, N., Kelepertzis, E., Tziritis, E., & Kelepertsis, A. (2015). Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. Journal of Geochemical Exploration, 159, 79–92. https://doi.org/10.1016/j.gexplo.2015.08.007.
Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. Source: Journal of the American Statistical Association (Vol. 58).
Wen, B., Zhou, J., Zhou, A., Liu, C., & Xie, L. (2016). Sources, migration and transformation of antimony contamination in the water environment of Xikuangshan, China: evidence from geochemical and stable isotope (S, Sr) signatures. The Science of the Total Environment, 569-570, 114–122. https://doi.org/10.1016/j.scitotenv.2016.05.124.
Wilbur, S., Ingerman, L., Citra, M., Osier, M., & Wohlers, D. (2000). Toxicological profile for chromium. Atlanta: Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp7.pdf. Accessed 12 Apr 2019
World Health Organization (WHO) (2017) Guidelines for drinking water quality. World Health Organization Geneva, 4th edition
Zasoski, R. J., & Fendorf, S. E. (1992). Chromium(III) oxidation by Δ-manganese oxide (MnO2). 1. Characterization. Environmental Science and Technology. https://doi.org/10.1021/es00025a006
Zhang, W., Singh, P., Paling, E., & Delides, S. (2004). Arsenic removal from contaminated water by natural iron ores. Minerals Engineering, 17, 517–524. https://doi.org/10.1016/j.mineng.2003.11.020.
Zhang, Y., Li, F., Zhang, Q., Li, J., & Liu, Q. (2014). Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. The Science of the Total Environment, 490, 213–222. https://doi.org/10.1016/j.scitotenv.2014.05.004.
Zhitkovich, A. (2011). Chromium in drinking water: sources, metabolism, and cancer risks. Chemical Research in Toxicology, 24, 1617–1629. https://doi.org/10.1021/tx200251t.
Acknowledgments
Constructive suggestions by an anonymous reviewer are highly acknowledged. We thank Dr. Yu-Pin Lin for editorial handling. This research was carried out within the framework of the Research Project NTUA 623147
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasileiou, E., Papazotos, P., Dimitrakopoulos, D. et al. Expounding the origin of chromium in groundwater of the Sarigkiol basin, Western Macedonia, Greece: a cohesive statistical approach and hydrochemical study. Environ Monit Assess 191, 509 (2019). https://doi.org/10.1007/s10661-019-7655-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7655-1