Abstract
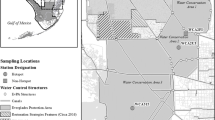
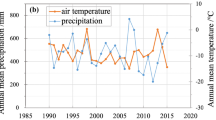
Concentrations of mercury were determined in above- and below-ground tissues of dominant plant species, as well as soils, in the wetlands of Lake Maurepas, Louisiana. Indicators of wetland soil biogeochemical status, such as soil redox potential, pore-water nutrient concentrations, and pore-water total sulfides, were also determined. Total mercury concentrations in plant tissues were within the typical range for vegetation not exposed to mercury contamination. Similarly, total mercury concentrations in soils were typical of uncontaminated wetlands within this geographic region. Soil methyl mercury levels in this study are slightly lower than those reported in other studies of nearby wetlands. This may reflect the less extensive geographic sampling in this study, or the low water levels in the Lake Maurepas system immediately prior to and during this study, which would have altered soil biogeochemical status. This is corroborated by measurements of soil redox potential and soil pore-water nitrogen and sulfur constituents conducted during this study that suggest minimal sulfate reduction was occurring in surficial soils. This study indicates that the wetlands surrounding Lake Maurepas are typical of many uncontaminated oligohaline wetlands in the southeastern U.S. in regard to mercury concentrations.
Similar content being viewed by others
References
APHA, American Public Health Association (2005). Standard methods for the examination of water and wastewater (21st ed., p. 1368). Washington: American Water Works Association, Water Pollution Control Federation, and the American Public Health Association.
Barbour, M. G., Burk, J. H., Pitts, W. D., Gilliam, F. S., & Schwartz, M. W. (1999). Terrestrial plant ecology (3rd ed.). Menlo Park: Benjamin Cummings.
Benoit, J., Gilmour, C. C., Mason, R. P., & Heyes, A. (1999). Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environmental Science and Technology, 33, 951–957.
Benoit, J. M., Gilmour, C. C., Heyes, A., Mason, R. P., & Miller, C. L. (2003). Geochemical and biological controls over methylmercury production and degradation in aquatic systems. In Y. Cai, & O. C., Braids (Eds.), Biogeochemistry of environmentally important trace elements. ACS symposium series (Vol. 835, p. 262).
Bloom, N. S. (1989). Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromotography with cold vapour atomic fluorescence detection. Canadian Journal of Fisheries and Aquatic Science, 46, 1131–1140.
Breteler, R. J., Valiela, I., & Teal, J. M. (1981). Bioavailability of mercury in several North-eastern U.S. Spartina ecosystems. Estuarine, Coastal and Shelf Science, 12, 155–166.
Canaario, J., Caetano, M., Vale, C., & Cesaario, R. (2007). Evidence for elevated production of methylmercury in salt marshes. Environmental Science and Technology, 41, 7376–7382.
Capiomont, A., Piazzi, L., & Pergent, G. (2000). Seasonal variations of total mercury in foliar tissues of Posidonia oceanic. Journal of the Marine Biological Association of the UK, 80, 1119–1123.
Compeau, G. C., & Bartha, R. (1984). Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology, 50, 498–502.
Delaune, R. D., Crozier, C. R., & Devai, I. (2002). Sulfate reduction in Louisiana marsh soils of varying salinity. Communications in Soil Science and Plant Analysis, 33(1&2), 79–94.
Delaune, R. D., Gambrell, R. P., Devai, I., Jugsujinda, A., & Kongchum, M. (2009). Total Hg and methyl Hg distribution in sediments of selected Louisiana water bodies. Journal of Environmental Science and Health, Part A: Toxic-Hazardous Substances and Environmental Engineering, 44, 557–567.
Delaune, R. D., Gambrell, R. P., Jugsujinda, A., Devai, I., & Hou, A. (2008). Total Hg, methyl Hg and other toxic heavy metals in a northern Gulf of Mexico estuary: Louisiana Pontchartrain basin. Journal of Environmental Science and Health, Part A Toxic/Hazardous Substances and Environmental Engineering, 43, 1006–1015.
Doumlele, D. G. (1981). Primary production and seasonal aspects of emergent plants in a tidal freshwater marsh. Estuaries, 4, 139–142.
Dupre, T. P., Granier, T. J., Keife, S., Marino, R., O’Rourke, S., Partridge, C., et al. (1999). Variation of mercury concentration in fish taken from Lake Boeuf, southeastern Louisiana. Microchemical Journal, 61, 156–164.
Eisler, R. (2006). Mercury hazards to living organisms (p. 336). Boca Raton: Taylor and Francis Group.
Gilmour, C. C., Henry, E. A., & Mitchell, R. (1992). Sulfate stimulation of mercury methylation in freshwater sediments. Environmental Science and Technology, 26, 2281–2287.
Girden, E. R. (1992). ANOVA repeated measures (p. 77). Newbury Park: Sage.
Grace, J. B., & Wetzel, R. G. (1981). Habitat partitioning and competitive displacement in cattails (Typha): Experimental field studies. The American Naturalist, 118, 463–474.
Guentzel, J. L. (2009). Wetland influences on mercury transport and bioaccumulation in South Carolina. Science of the Total Environment, 407, 1344–1353.
Hall, B. D., Aiken, G. R., Krabbenhoft, D. P., Marvin-DiPasquale, M., Swarzenskif, C. M., Korthals, E. T., et al. (2008). Seasonal and spatial variations in mercury methylation and demethylation in an oligotrophic lake. Applied and Environmental Microbiology, 53, 2397–2404.
Hall, B. D., & St Louis, V. (2004). Methylmercury and total mercury in plant litter decomposing in upland forests and flooded landscapes. Environmental Science and Technology, 38, 5010–5021.
Han, F. X., Shiyab, S., Chen, J., Su, Y., Monts, D. L., Waggoner, C. A., et al. (2008). Extractability and bioavailability of mercury from a mercury sulfide contaminated soil in Oak Ridge, Tennessee, USA. Water, Air, and Soil Pollution, 194, 67–75.
Hester, M. W., Willis, J. M., Kaller, M. J., Mayence, C. E., & Crane, T. E. (2005). Ecology and restoration potential of the Manchac area wetlands: Constraints on plant establishment and community composition. Phase I Final Report. US EPA/SRPP.
Hollweg, T. A., Gilmour, C. C., & Mason, R. P. (2009). Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Marine Chemistry, 114, 86–101.
Howard, R. J., & Mendelssohn, I. A. (1999). Salinity as a constraint on growth of oligohaline marsh macrophytes. I. Species variation in stress tolerance. American Journal of Botany, 86, 785–794.
Kongchum, M., Devai, I., DeLaune, R. D., & Jugsujinda, A. (2006). Total mercury and methylmercury in freshwater and salt marsh soils of the Mississippi river deltaic plain. Chemosphere, 63, 1300–1303.
Korthals, E. T., & Winfrey, M. R. (1987). Seasonal and spatial variations in mercury methylation and demethylation in an oligotrophic lake. Applied and Environmental Microbiology, 53, 2397–2404.
Kubanek, J., Fenical, W., Hay, M. E., Brown, P. J., & Lindquist, N. (2000). Two antifeedant lignans from the freshwater macrophyte Saururus cernuus. Phytochemistry, 54, 281–287.
Lambertsson, L., & Nilsson, M. (2006). Organic material: The primary control on mercury methylation and ambient methyl mercury concentrations in estuarine sediments. Environmental Science and Technology, 40, 1822–1829.
Lacerda, L. D., & Fitzgerald, W. F. (2001). Biogeochemistry of mercury in wetlands. Wetlands Ecology and Management, 9, 291–293.
Landers, J. F., Gubala, C., Monetti, M., Lasorsa, B. K., & Martinson, J. (2005). Mercury in vegetation and lake sediments from the U. S. arctic. Water, Air, and Soil Pollution, 80, 1573–2932.
Lewis, M., & Chancy, C. (2008). A summary of total mercury concentrations in flora and fauna near common contaminant sources in the Gulf of Mexico. Chemosphere, 70, 2016–2024.
Mailman, M., & Bodaly, R. A. (2005). Total mercury, methyl mercury, and carbon in fresh and burned plants and soil in Northwestern Ontario. Environmental Pollution, 138, 161–166.
McKee, K. L., Mendelssohn, I. A., & Hester, M. W. (1988). A reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. American Journal of Botany, 75, 1352–1359.
Meert, D. R., & Hester, M. W. (2009). Response of a Louisiana oligohaline marsh plant community to nutrient availability and disturbance. Journal of Coastal Research, 54, 174–185.
Merritt, K. A., & Amirbahman, A. (2009). Mercury methylation dynamics in estuarine and coastal marine environments—a critical review. Earth-Science Reviews, 96, 54–66.
Moore, T. R., Bubier, J. L., Heyes, A., & Flett, J. (1995). Methyl and total mercury in boreal wetland plants, experimental lakes area, Northwestern Ontario. Journal of Environmental Quality, 24, 845–850.
National Science and Technology Council Committee (2004). National Science and Technology Council Committee on the Environmental and Natural Resources, N. Methylmercury in the Gulf of Mexico: State of Knowledge and Research Needs.
O’Rourke, S., Gauthreaux, K., Noble, C. O., Sneddon, J., & Beck, J. N. (2001). Mercury in sediments collected at the Sabine National Wildlife Refuge Marsh reclamation site in southwest Louisiana. Microchemical Journal, 70, 1–5.
Patrick, W. H., Gambrell, R. P., & Faulkner, S. P. (1996). Redox measurements of soils. In D. L. Sparks, (Ed.), Methods of soil analysis, chemical methods (pp. 417–436). Madison: Soil Science Society of America.
Rajbhandari, I., Takamatsu, S., & Nagle, D. G. (2001). A new dehydrogeranylgeraniol antioxidant from Saururus cernuus that inhibits intracellular reactive oxygen species (ROS)-catalyzed oxidation within HL-60 cells. Journal of Natural Products, 63, 693–695.
Ravichandran, M. (2004). Interactions between mercury and dissolved organic matter—a review. Chemosphere, 55, 319–331.
Reddy, K. R., & DeLaune, R. D. (2008). Biogeochemistry of wetlands: Science and applications (p. 774). Boca Raton: CRC.
Rencz, A. N., O’Driscoll, N. J., Hall, G. E. M., Peron, T., Telmer, K., & Burgess, N. M. (2004). Spatial variation and correlations of mercury levels in the terrestrial and aquatic components of a wetland dominated ecosystem: Kejimkujik Park, Nova Scotia, Canada. Water, Air, and Soil Pollution, 143, 271–288.
Sasser, C. E., Gosselink, J. G., & Shaffer, G. P. (1991). Distribution of nitrogen and phosphorus in a Louisiana freshwater floating marsh. Aquatic Botany, 41, 317–331.
Shaffer, G. P., Perkins, T. E., Hoeppner, S., Howell, S., Benard, H., & Parsons, A. C. (2003). Ecosystem health of the Maurepas Swamp: Feasibility and projected benefits of a freshwater diversion (p. 105). Final Report. Environmental Protection Agency.
Skyllberg, U., Qian, J., Frech, W., Xia, K., & Bleam, W. F. (2003). Distribution of mercury, methyl mercury and organic sulphur species in soil, soil solution and stream of a boreal forest catchment. Biogeochemistry, 64, 53–76.
Soil Testing and Plant Analysis Council (2000). Soil analysis: Handbook of reference methods (p. 247). Boca Raton: CRC.
Spalding, E. A., & Hester, M. W. (2007). Interactive effects of hydrology and salinity on oligohaline plant species productivity: Implications of relative sea-level rise. Estuaries and Coasts, 30, 214–225.
St. Louis, V. L., Rudd, J. W. M., Kelly, C. A., Beaty, K. G., Bloom, N. S., & Flett, R. J. (1994). Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 51, 1065–1076.
Swarzenski, C. M., Doyle, T. W., Fry, B., & Hargis, T. G. (2008). Biogeochemical response of organic-rich freshwater marshes in the Louisiana delta plain to chronic river water influx. Biogeochemistry, 90, 49–63.
Von Ende, C. N. (2001). Repeated-measure analysis: Growth and other time-dependent measures. In S. M. Scheiner, & J. Gurevitch (Eds.), Design and analysis of ecological experiments (p. 415). Oxford: Oxford University Press.
Whigham, D. F., & Simpson, R. L. (1978). The relationship between aboveground and belowground biomass of feshwater tidal wetland macrophytes. Aquatic Botany, 5, 355–364.
Windham, L., Weis, J. S., & Weis, P. (2001). Patterns and processes of mercury release from leaves of two dominant salt marsh macrophytes. Phragmites australis and Spartina alterniflora. Estuaries, 24, 787–795.
Windham, L., Weis, J. S., & Weis, P. (2003). Uptake and distribution of metals in two salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Estuarine,Coastal and Shelf Science, 56, 63–72.
Windham, L., Weis, J. S., & Weis, P. (2004). Metal dynamics of plant litter of Spartina alterniflora and Phragmites australis in metal-contaminated salt marshes. Part 1. Patterns of decomposition and metal uptake. Environmental Toxicology & Chemistry, 23, 1520–1528.
Windham-Myers, L., Marvin-DiPasquale, M., Krabbenhoft, D. P., Agee, J. L., Cox, M. H., Heredia-Middleton, P., et al. (2009). Experimental removal of macrophyte vegetation in four wetlands leads to decreased methylmercury production and concentrations in surface sediments. Journal of Geophysical Research: Biogeochemistry, 114, 1–14.
Windom, H., Gardner, W., Stephens, J., & Taylor, F. (1976). The role of methylmercury production in the transfer of mercury in a salt marsh ecosystem. Estuarine and Coastal Marine Science, 4, 579–583.
Yu, K., Delaune, R. D., Devai, I., Tao, R., & Jugsujinda, A. (2008). Total and methyl mercury in wetland soils and sediments of Louisiana’s Pontchartrain Basin (USA). Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances and Environmental Engineering, 43, 1657–1662.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willis, J.M., Gambrell, R.P. & Hester, M.W. Mercury concentrations in oligohaline wetland vegetation and associated soil biogeochemistry. Environ Monit Assess 181, 373–383 (2011). https://doi.org/10.1007/s10661-010-1835-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-010-1835-3