Abstract
Mercury (Hg) is a toxicant of concern, particularly in aquatic food webs. Mercury can move to terrestrial systems through consumption of aquatic prey or emergence of insects with aquatic larval phases. The possible movement of Hg from sediments to wetland plants and into terrestrial food webs though primary consumers has received less attention. We investigated differences and correlations in Hg in soil, wood and leaves of willows (Salix caroliniana) and folivorous beetles from a wetland with enhanced levels of Hg. Further, we compared samples from tree islands that had enriched Hg in soil through bird guano with control islands. Hg in any sample type did not correlate with Hg in any other sample type from the same island. We found higher [Hg] in soils and significantly higher [Hg] in leaves from colony islands, while [Hg] in beetles appeared to be higher in control islands. In any case, despite comparatively high [Hg] in soil and leaves, Hg in folivorous beetles was below detection levels and lower than that reported from other studies. We conclude that movement of Hg from wetland trees to terrestrial food webs through wetland vegetation is negligible in this ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a long-lived toxicant in soil and sediments that bioaccumulates and biomagnifies in food webs, particularly aquatic ones (Scheuhammer et al. 2007). In aquatic systems, Hg is also often in its much more toxic methylated form, due to the action of several microorganism (Zillioux et al. 1993; Tang et al. 2020; Peterson et al. 2020). The movement of aquatic mercury to terrestrial systems through emergence of the aerial or terrestrial phase of aquatic invertebrates and other consumption of aquatic prey by terrestrial organisms is well known (Cristol et al. 2008; Gann et al. 2015; Williams et al. 2017). In plants, Hg in leaves comes mostly from atmospheric deposition (Peckham et al. 2019) and there is evidence of the movement of Hg in leaves and its bioaccumulation through terrestrial food-webs (Rimmer et al. 2010; Luo et al. 2020). However, movements and biomagnifcation of Hg through arboreal food webs is poorly explored. Plants also uptake Hg from soils, and though accumulation tends to be in roots (Wang and Greger 2001; Rodríguez-Alonso et al. 2019), a fraction of it is mobilized and leaf Hg concentrations ([Hg] hereafter) can be influenced by substrate Hg. Yet, plants tend to mobilize less Hg to leaves as they age (Patra and Sharma 2000; Rodríguez-Alonso et al. 2019). Further, wetland plants or plants growing close to polluted wetlands tend to have higher [Hg] in leaves (Moore et al. 1995; Patra and Sharma 2000; Wang et al. 2020) and in contaminated areas wetland plants such as rice can be a source of exposure (Abeysinghe et al. 2017). Therefore, wetland plants could play a role as a secondary pathway of Hg into the food web, particularly in wetlands with high [Hg].
Ecological dynamics can create Hg hotspots within polluted ecosystems. Colonial birds breeding in high density aggregations can concentrate Hg from the feeding area around their colonies though guano (Zhu et al. 2014). In the Florida Everglades, although long legged wading birds (herons, egrets, ibises, storks and spoonbills) redistributed only a small fraction of the Hg in the entire system through guano, annual deposition in colony islands through guano was estimated to be eight times atmospheric deposition (Zhu et al. 2014). In active breeding colonies soil [Hg] was on average 65% higher than in non-colony (control) islands (234 µg/kg compared to 142 µg/kg dw), with several tree islands having more than 400 µg/kg Hg in soil (Zhu et al. 2014).
The influence of soil Hg enrichment by bird guano on wetland vegetation [Hg] and its possible movement through primary consumers into the food-web remain unknown. We investigated [Hg] in soil, wood and leaves of willows and primary consumers (herbivorous coleopterans) in tree islands of the Everglades. We predicted that [Hg] in plant material of colony islands would be higher than in control islands, correlated with higher [Hg] in soil, and that it would move in significantly higher amounts into within-island food webs through higher [Hg] in primary consumers.
Materials and methods
Sample collection and Hg determination
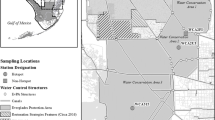
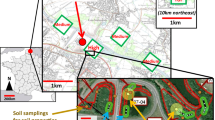
Between 17 April and 9 May 2019 we sampled 12 tree islands within Water Conservation Area 3A of the Everglades (Dade and Broward counties, Florida, USA; Fig. S1). Breeding colonies of wading birds are generally located on tree islands set widely apart (cf 2–15 km) within a vast expanse of graminoid marsh. We classified islands as colony or control islands using information from monthly (January to July) systematic, 100% coverage aerial and ground surveys to detect and quantify breeding pairs in breeding colonies between 1994 and 2018 (Frederick et al. 1996; Frederick and Ogden 2003; Williams et al. 2008; Zabala et al. 2020). Control islands were those in which no breeding colony (> 3 nesting pairs of any wading bird species) was detected in the 1994–2018 period. We defined colony islands as those that harbored breeding activity in > 3 years within the study period. 2019 was an atypical year with repeated events of rainfall in the early months of the breeding season resulting in poor breeding conditions for wading birds. In consequence, no breeding colonies were active before or during the sampling period and all Hg values observed represent legacies from previous years. Only one of the colony islands sampled (Donut Mel) had substantial presence of birds in 2019: several anhinga (Anhinga anhinga) nests were active during sampling visits in sampling area. We sampled five control islands and seven colony islands. In each of these islands, we defined sampling areas as a 5 m radius from a randomly selected central point. All sampling areas were within the regularly flooded band of willow (Salix caroliniana > 80% willow canopy cover) where wading birds typically nest. Restricting samples to willow vegetation areas also reduced problems related to specific variation in Hg accumulation among different tree species (Laacouri et al. 2013).
Within sampling areas, we collected samples of soil, plants and primary leaf-consumers. We collected the top 10 cm of soil from 5–6 randomly chosen points within the sampling area and merged them into a single soil sample per site. We sampled only from areas without surface water and scraped the surface clean of recent large (> 5 cm2) organic debris before collection. To sample living wood stems, we selected four upright willow trunks growing within each sampling area and cut Sects. 30–45 cm long and of 2–2.5 cm diameter. We also collected twigs (0.3–0.5 cm diameter) from another six willows, and collected 10 samples of leaves from these ten willows (4 branches + 6 twigs). In leaf sample collection, we discarded buds and new leaves (growing leaves that were soft and lighter in color) and only collected full-grown leaves. Finally, in each sampling area we randomly selected four 3 × 3 m square plots and sampled the willow canopy for arthropods using a leaf vacuum-blower (Stihl SH 56 C-E) in vacuum mode with a stocking fitted in the muzzle to collect samples and an organdy mesh below to prevent aspired items from reaching the shredder. We vacuumed each area thoroughly for 4 min or until all the branches and canopy in the area had been sampled. In the field, we stored different samples and samples from different areas in sealed plastic bags or jars.
Once out of the field, we stored soils in waterproof bags in a cool environment and allowed them to air dry. We thoroughly washed branches and discarded sections with lichens, then we stored branch sections in paper envelopes in a cool and shady area and allowed them to air dry. We discarded leaves with non-plant material (leaf galls, spider nests, invertebrate eggs, feces…), and those heavily damaged by herbivory or showing discoloration or spots that could be indicative of the presence of other organisms. We thoroughly washed remaining leaves under water and stored them in paper envelopes grouped by tree (i.e., ten envelopes per island) in a cool and dark place. We stored invertebrate samples in a cooler with ice in the field and once back in the field house we froze them for at least 24 h to humanely kill specimens. Next, we allowed the samples to defrost for at least 6 h and then collected arthropods from the stockings and stored them frozen in paper envelopes.
Soils of tree islands of the Everglades are primarily peat and water saturated. Therefore, prior to processing soil samples for [Hg] we dried soil samples in an oven at 45 °C and with a forced air flow for 48 h. We then separated fine materials (< 2 mm diameter and < 5 mm length) into paper envelopes. We thoroughly washed branches and leaves again with deionized water and allow them to air dry. Next, we used plant mills to grind branches and leaves into fine material (< 2 mm) and stored them in paper envelopes. Some leaf-samples consisted of very few leaves from the same tree and a considerable amount of plant material was lost in the plant mills. Thus, in some occasions we had to combine samples from two or more of the ten leaf-samples from the same island to ensure enough material for analyses. To avoid cross contamination, between samples we thoroughly cleaned the mill and sieves with compressed air. We also discarded the first material coming out of the mill in each occasion. Arthropod canopy samples were dominated by a single coleopteran species, the cottonwood leaf beetle (Chrysomela scripta: Fig. 1). The cottonwood leaf beetle is native to the United States and both larvae and adults feed on leaves, particularly poplar (Populus spp.) but also willow (Orians et al. 1997; Coyle et al. 2001). Thus, and to minimize variance arising from possible interspecific variability in Hg accumulation, we focused on cottonwood leaf beetles. Prior to Hg estimation, we washed beetles in deionized water and allowed them to air dry. Next, we oven dried them at 45 °C for > 48 h, created composite samples by merging 3–5 beetles from the same island, and ground them using a mortar and a pestle, and stored dry ground beetles in paper envelopes. To avoid cross contamination, after grinding beetles from each island and, we cleaned the pestle and mortar with deionized water and with acetone and allow it to air dry.
Boxplot with the distribution of [Hg] in each sample type in colony and control islands: A soils, B Wood, C leaves and D beetles. Note different vertical axis scales and units in plots. Hg in coleopteran samples were below detection limits and results should be regarded with care. Thick central lines show the median and boxes include the range between 0.25 and 0.75 quartiles. Blue points show values for individual observations and have been jittered horizontally and to a lesser degree vertically to enhance visualization. We show the p value for each pairwise comparison (Table 1). The image in plot D is of C. scripta, the beetle used in analyses. Note that concentration units differ among plots
Immediately prior to Hg determination we oven-dried all final materials (fine soil material, ground leaves, ground wood dust, and ground beetle composites) within their envelopes at 60 °C for at least 48 h to standardize hydration level of samples. We oven dried all materials at < 65 °C because it effectively eliminates water while effectively conserving Hg compared to air drying or drying at higher temperatures (Hojdová et al. 2015).
We measured total [Hg] using a Direct Mercury Analyzer (Milestone DMA 80). In each batch of 20–40 samples we included at least two blanks, 10–50% replicates depending on sample material availability, and 2–4 standard reference samples (DOLT-2 dogfish liver, TORT-2 lobster hepatopancreas and DORM-2 dogfish muscle). Recovery rates (± SD) were 101.93% (± 1.56) for DOLT-2 (N = 24), 109.82% (± 3.53) for TORT-2 (N = 23) and 95.57% (± 1.71) for DORM-2 (N = 14).
Statistical analyses
We assessed differences in Hg between control and colony islands using Generalized Linear Models (GLMs) with the identity link function and a Gaussian distribution of error (Crawley 2007) in which the concentration of Hg ([Hg]) hereafter) was the response variable and island type (colony vs control) a categorical predictor.
We assessed within-island correlation of [Hg] in different samples (e.g. soil [Hg] and leaf [Hg]) using GLMs with the identity link function and a Gaussian distribution of error and average [Hg] in each sample type as response and predictor variables.
All descriptive statistics are reported as mean values and one standard deviation (mean ± SD) unless otherwise stated.
We ran all models using R (R Core Team 2021) and produced the plot using ggplot2 (Wickham 2009).
Results
We found non-significantly higher [Hg] in soils from colony islands and significantly higher [Hg] in leaves from colony islands. However, that did not translate to primary consumers, as, contrary to our prediction, beetles from colony islands had significantly lower [Hg]. Further, [Hg] in different sample types did not correlate within islands suggesting a lack of passage of Hg within the trophic web.
Soil [Hg] was on average 27% higher in colony than control islands (0.148 ± 0.053 and 0.117 ± 0.022 mg Hg /kg respectively; Fig. 1), but that difference was not statistically significant (p = 0.24; N = 12: Table 1). Average Hg in wood from colonies was 176% that of wood from control islands (0.312 ± 0.671 and 0.113 ± 0.355 µg Hg /kg respectively; Fig. 1). However, both samples were strongly influenced by a few branches with proportionately very high [Hg] and the differences between island types were not significant (p = 0.25; Table 1). Leaves in colony islands had on average 22% higher [Hg] than leaves from control islands (0.013 ± 0.005 and 0.011 ± 0.004 mg Hg /kg respectively), and the difference was statistically significant (p = 0.011; Table 1).
Beetle [Hg] was uniformly low except for a single outlier apparently biased with residual Hg in the burning chamber from a standard reference material burnt before it. Since the difference between standard material and all other beetle values was seven orders of magnitude, we discarded this single sample as an outlier. Disregarding that particular value, Hg in beetles from control islands was 66% higher than in colonies (0.200 ± 0.065 and 0.333 ± 0.1187 ng Hg /Kg in colonies and controls respectively). However, Hg in beetle samples was near or below detection limits of the DMA-80 (0.0003 ng) and the reported difference should be regarded with caution. Values for each of the four sample types (soil, wood, leaves and beetles) varied among islands independently of values in other sample types. In addition to results of linear associations reported in Table 1, we also tested the logarithmic and exponential shapes. None of the analyses resulted in significant associations (Table S1).
Discussion
We found higher [Hg] in soil from colony islands than in soil from control islands but that difference was not significant, possibly as a consequence of reduced sample size rather than lack of real differences. Our values were within the range though somewhat lower than those previously reported for Everglades tree islands, (Zhu et al. 2014). The islands included in this study lay on a gap in the distribution of islands sampled by Zhu et al. (2014), but within the area (Water Conservation Area 3A) where they reported the lowest soil Hg values. Our values for wood (0.3 and 0.1 ng/g) were below values reported in wood for several hardwoods and conifers from the northeast USA (0.5–3.5 ng/g; Yang et al. 2018). They were also below values for branches reported of several species across the USA (1–57 ng/g) despite our values of soil [Hg] (98–240 ng/g) being in the range and generally higher than soil [Hg] in the sites included in that study (9–251 ng/g; Obrist et al. 2011). Comparing to other Salix species, [Hg] in wood of Salix caroliniana from tree islands in this study was lower than values reported for wood from Hg contaminated sites (forests close to coal burning plants and brownfields where Hg using industries existed in the past) and conservation areas used as reference sites (2.0 and 2.2 ng/g) even though those sites had lower soil [Hg] (28.8 and 63.6 ng/g respectively; Siwik et al. 2010) than those in our study areas. Leaves, on the other hand, were within the mid-low range of Hg values reported for foliage in other studies (Rea et al. 2002; Siwik et al. 2009; Yang et al. 2018). Leaf [Hg] values are known to vary among species (Siwik et al. 2009), but our values were higher than average values reported for willow (Salix spp) leaves from control and polluted areas referred above (7.4 and 8.9 ng/g respectively; Siwik et al. 2010). Our values for Hg in beetles were negligible and generally below detection limits. In any case, considering the average sample weight used (0.048 g) and the detection limit of the DMA 80 (0.0003 ng) [Hg], Hg must have been below 0.1 ng/g or 10–4 mg/kg in every composite sample. That is less than reported values for any insect samples from polluted or control areas so far, including forest, rice paddies, rivers and wetlands (Cristol et al. 2008; Rimmer et al. 2010; Obrist et al. 2011; Zhang et al. 2012; Wang et al. 2013; Ortiz et al. 2015; Abeysinghe et al. 2017; Udodenko et al. 2019; Luo et al. 2020). Leaf Hg values suggest that folivorous species are exposed to more Hg in diet than in other study areas and, within our study site, higher in colony than in control islands.
Studies in magnification through folivory are scarce. A study in a montane terrestrial food-web where the main source of Hg was atmospheric deposition reported 0.008 mg/Kg Hg in deciduous foliage—similar to our values, which was biomagnified to 0.019–0.034 mg/Kg Hg in folivorous insects (Rimmer et al. 2010). Another study reported Hg biomagnification in a terrestrial pine forest food-web from 0.011 ± 0.0077 mg/kg Hg in pine needles, similar to our values, to 0.027 ± 0.013 mg/kg Hg in caterpillars feeding on needles (Luo et al. 2020). Both studies showed obvious biomagnification, which was in clear contrast with our results. Possible explanations for the low levels of Hg in the beetles we studied could be (1) that Hg in willow leaves is bound to parts not absorbed by insects during digestion, (2) that Hg in leaves is in a chemical form that is not readily assimilated by insects, (3) due to possible physiological particularities of the coleopteran species studied, or (4) a consequence of beetle movement and dispersal, Hg in captured beetles represented exposure from other environments. In any case, high Hg levels are common in many other areas of the Everglades (Zabala et al. 2020; Janssen et al. 2022) and beetles would have to move long distances (cf 20 km) to arrive from low exposure areas.
In any case, our findings do not support the idea that plants are taking up Hg from soil and causing Hg to enter into the terrestrial or at least arboreal food web. Higher [Hg] in leaves in colonies than in control islands could be compatible with an explanation of atmospheric uptake (Peckham et al. 2019), possibly through a mechanism of higher Hg0 in the air in colony islands resulting from higher emissions of the local water and vegetation. In addition to higher local Hg sources, colony islands may have vegetation that emits more of the Hg that is present. Wading bird guano increases phosphorus in the highly oligotrophic Everglades soils (Irick et al. 2015), and one of the consequences of enriched phosphorus in the Everglades is the substitution of sawgrass (Cladium jamaicense) by cattail (Typha spp.) stands (Turner et al. 1999). A study assessing Hg° transpiration through vegetation in our study area found that cattail transpired twice as much gaseous elemental Hg to the atmosphere than sawgrass over comparable periods (Lindberg et al. 2002).
The lack of association between soil and plant [Hg] from the same sampling areas and the low [Hg] observed in plants, compared with those of plant material from other areas, also supports the idea that soil Hg is not incorporated into plant tissues in significant amounts. Hg in willow species tends to concentrate in roots with only a small fraction mobilized to growing tissues (Wang and Greger 2001). Our results suggest that even in high soil [Hg] environments the effect of that accumulation on willow leaves is negligible. Finally, adult insects whose larvae and adult forms feed on foliage and presumably grew on local willow leaves had only trace levels of Hg. Studies in folivorous insects (Rimmer et al. 2010; Luo et al. 2020) and large mammal herbivores (Gnamuš et al. 2000; Pokorny and Ribaric-Lasnik 2002; Durkalec et al. 2015) reported relevant Hg uptake and accumulation through food. This is despite plant foods not being active Hg accumulators generally and with plant [Hg] values smaller than ours (Gnamuš et al. 2000). However, our results suggest that high or enriched Hg levels in wetlands may not enter the terrestrial food web through primary consumers of wetland tree foliage.
Data availability
When published data will be uploaded to a public repository.
Code availability
Not applicable.
References
Abeysinghe KS, Qiu G, Goodale E et al (2017) Mercury flow through an Asian rice-based food web. Environ Pollut 229:219–228. https://doi.org/10.1016/j.envpol.2017.05.067
Coyle DR, Mcmillin JD, Hall RB, Hart ER (2001) Cottonwood leaf beetle (Coleoptera: Chrysomelidae) larval performance on eight populus clones. Environ Entomol 30:748–756. https://doi.org/10.1603/0046-225x-30.4.748
Crawley MJ (2007) The R Book. John Wiley & Sons Ltd, Chichester
Cristol DA, Brasso RL, Condon AM et al (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:35–36. https://doi.org/10.1126/science.1154082
Durkalec M, Szkoda J, Kolacz R et al (2015) Bioaccumulation of lead, cadmium and mercury in roe deer and wild boars from areas with different levels of toxic metal pollution. Int J Environ Res 9:205–212
Frederick PC, Ogden J (2003) Monitoring wetland ecosystems using avian populations: seventy years of surveys in the Everglades. In: Bush D, Trexler JC (eds) Monitoring ecosystems: interdisciplinary approaches for evaluating ecoregional initiatives. Island Press, Washington, pp 321–340
Frederick PC, Towles T, Sawicki RJ, Bancroft TG (1996) Comparison of aerial and ground techniques for discovery and census of wading bird (Ciconiiformes) nesting colonies. Condor 98:837–841
Gann GL, Powell CH, Chumchal MM, Drenner RW (2015) Hg-contaminated terrestrial spiders pose a potential risk to songbirds at Caddo lake (Texas/Louisiana, USA). Environ Toxicol Chem 34:303–306. https://doi.org/10.1002/etc.2796
Gnamuš A, Byrne AR, Horvat M (2000) Mercury in the soil-plant-deer-predator food chain of a temperate forest in Slovenia. Environ Sci Technol 34:3337–3345. https://doi.org/10.1021/es991419w
Hojdová M, Rohovec J, Chrastný V et al (2015) The influence of sample drying procedures on mercury concentrations analyzed in soils. Bull Environ Contam Toxicol 94:570–576. https://doi.org/10.1007/s00128-015-1521-9
Irick DL, Gu B, Li YC et al (2015) Wading bird guano enrichment of soil nutrients in tree islands of the Florida Everglades. Sci Total Environ 532:40–47. https://doi.org/10.1016/j.scitotenv.2015.05.097
Janssen SE, Tate MT, Poulin BA et al (2022) Decadal trends of mercury cycling and bioaccumulation within Everglades National Park. Sci Total Environ 838:156031. https://doi.org/10.1016/j.scitotenv.2022.156031
Laacouri A, Nater EA, Kolka RK (2013) Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, USA. Environ Sci Technol 47:10462–10470. https://doi.org/10.1021/es401357z
Lindberg SE, Dong W, Meyers T (2002) Transpiration of gaseous elemental mercury through vegetation in a subtropical wetland in Florida. Atmos Environ 36:5207–5219. https://doi.org/10.1016/S1352-2310(02)00586-1
Luo K, Xu Z, Wang X et al (2020) Terrestrial methylmercury bioaccumulation in a pine forest food chain revealed by live nest videography observations and nitrogen isotopes. Environ Pollut 263:114530
Moore TR, Bubier JL, Heyes A, Flett RJ (1995) Methyl and total mercury in boreal wetland plants, experimental lakes area, Northwestern Ontario. J Environ Qual 24:845–850. https://doi.org/10.2134/jeq1995.00472425002400050007x
Obrist D, Johnson DW, Lindberg SE et al (2011) Mercury distribution across 14 US forests. Part I: spatial patterns of concentrations in biomass, litter, and soils. Environ Sci Technol 45:3974–3981. https://doi.org/10.1021/es104384m
Orians CM, Huang CH, Wild A et al (1997) Willow hybridization differentially affects preference and performance of herbivorous beetles. Entomol Exp Appl 83:285–294. https://doi.org/10.1023/A:1002985414639
Ortiz C, Weiss-Penzias PS, Fork S, Flegal AR (2015) Total and monomethyl mercury in terrestrial arthropods from the central California coast. Bull Environ Contam Toxicol 94:425–430. https://doi.org/10.1007/s00128-014-1448-6
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422. https://doi.org/10.1007/BF02868923
Peckham MA, Gustin MS, Weisberg PJ, Weiss-Penzias P (2019) Results of a controlled field experiment to assess the use of tree tissue concentrations as bioindicators of air Hg. Biogeochemistry 142:265–279. https://doi.org/10.1007/s10533-018-0533-z
Peterson BD, McDaniel EA, Schmidt AG et al (2020) Mercury methylation genes identified across diverse anaerobic microbial guilds in a eutrophic sulfate-enriched lake. Environ Sci Technol 54:15840–15851. https://doi.org/10.1021/acs.est.0c05435
Pokorny B, Ribaric-Lasnik C (2002) Seasonal variability of mercury and heavy metals in roe deer (Capreolus capreolus) kidney. Environ Pollut 117:35–46
R Core Team (2021) R: a language and environment for statistical computing. Vienna, Austria. https://www.R-project.org/
Rea AW, Lindberg SE, Scherbatskoy T, Keeler GJ (2002) Mercury accumulation in foliage over time in two northern mixed-hardwood forests. Water Air Soil Pollut 133:49–67. https://doi.org/10.1023/A:1012919731598
Rimmer CC, Miller EK, Faccio SD (2010) Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology 19:697–709. https://doi.org/10.1007/s10646-009-0443-x
Rodríguez-Alonso J, Sierra MJ, Lominchar MÁ, Millán R (2019) Effects of mercury on the germination and growth of Quercus ilex L. seedlings. Environ Sci Pollut Res 26:30930–30940. https://doi.org/10.1007/s11356-019-06186-8
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18. https://doi.org/10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2
Siwik EIH, Campbell LM, Mierle G (2009) Fine-scale mercury trends in temperate deciduous tree leaves from Ontario, Canada. Sci Total Environ 407:6275–6279. https://doi.org/10.1016/j.scitotenv.2009.08.044
Siwik EIH, Campbell LM, Mierle G (2010) Distribution and trends of mercury in deciduous tree cores. Environ Pollut 158:2067–2073. https://doi.org/10.1016/j.envpol.2010.03.002
Tang W-L, Liu Y-R, Guan W-Y et al (2020) Understanding mercury methylation in the changing environment: recent advances in assessing microbial methylators and mercury bioavailability. Sci Total Environ 714:136827. https://doi.org/10.1016/j.scitotenv.2020.136827
Turner AM, Trexler JC, Jordan CF et al (1999) Targeting ecosystem features for conservation: standing crops in the Florida Everglades. Conserv Biol 13:898–911. https://doi.org/10.1046/j.1523-1739.1999.97513.x
Udodenko YG, Seleznev DG, Prokin AA et al (2019) Mercury accumulation in adults of two large species of diving beetles (Coleoptera: Dytiscidae). Russ Entomol J 28:23–29. https://doi.org/10.15298/rusentj.28.1.04
Wang Y, Greger M (2001) Plant and environment interactions. J Environ Qual 33:1779–1785
Wang Q, Zhang Z, Zhou X, Xianguo L (2013) Mercury distribution and accumulation in typical wetland ecosys- tems of Sanjiang Plain. Northeast China 23:49–58. https://doi.org/10.1007/s11769-
Wang J, Shaheen SM, Anderson CWN et al (2020) Nano-activated carbon reduces mercury mobility and uptake by Oryza Sativa L: mechanistic investigation using spectroscopic and microscopic techniques. Environ Sci Technol. https://doi.org/10.1021/acs.est.9b05685
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Williams KA, Frederick PC, Kubilis PS, Simon JC (2008) Bias in aerial estimates of the number of nests in White Ibis and Great Egret colonies. J F Ornithol 79:438–447. https://doi.org/10.1111/j.1557-9263.2008.00197.x
Williams EB, Chumchal MM, Drenner RW, Kennedy JH (2017) Seasonality of odonate-mediated methylmercury flux from permanent and semipermanent ponds and potential risk to red-winged blackbirds (Agelaius phoeniceus). Environ Toxicol Chem 36:2833–2837. https://doi.org/10.1002/etc.3844
Yang Y, Yanai RD, Driscoll CT et al (2018) Concentrations and content of mercury in bark, wood, and leaves in hardwoods and conifers in four forested sites in the Northeastern USA. PLoS ONE 13:1–14. https://doi.org/10.1371/journal.pone.0196293
Zabala J, Trexler JC, Jayasena N, Frederick P (2020) Early breeding failure in birds due to environmental toxins: a potentially powerful but hidden effect of contamination. Environ Sci Technol 54:13786–13796. https://doi.org/10.1021/acs.est.0c04098
Zhang Z, Song X, Wang Q, Lu X (2012) Mercury bioaccumulation and prediction in terrestrial insects from soil in Huludao City, Northeast China. Bull Environ Contam Toxicol 89:107–112. https://doi.org/10.1007/s00128-012-0649-0
Zhu Y, Gu B, Irick DL et al (2014) Wading bird guano contributes to Hg accumulation in tree island soils in the Florida Everglades. Environ Pollut 184:313–319. https://doi.org/10.1016/j.envpol.2013.08.037
Zillioux EJ, Porcella DB, Benoit JM (1993) Mercury cycling and effects in freshwater wetladn ecosystems. Environ Toxicol Chem 12:2245–2264
Acknowledgements
We are grateful to Nicole D. Benda for her assistance with methods for sampling canopy arthropods. The C. scripta image in Fig. 1 was taken by Pavel Kirilov (St. Petersburg; Russia) and we downloaded it form Wikipedia and adapted it under the Creative Commons Attribution-Share Alike 2.0 Generic License. Soil and leaf images were download from SVG Silh and used under Creative Commons CC0 license. The wood image in Fig. 1 was modified from the “enjoy the wood” by Marko Velotskyi downloaded from Wikipedia under Creative Commons Attribution-Share Alike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-sa/4.0/. We are thankful to one anonymous reviewer for comments that improved the clarity and quality of the original version of this manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
PF and JZ designed the study. JZ performed sampling and analyses. JZ and PF wrote and edited the ms.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zabala, J., Frederick, P. Mercury movement from Hg-enriched wetland soils to arboreal food webs: a weak role for folivory. Wetlands Ecol Manage 31, 169–176 (2023). https://doi.org/10.1007/s11273-023-09908-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09908-5