Abstract
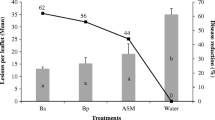
Some antagonistic bacteria contribute to the management of plant diseases by stimulating the host natural defense and/or by providing direct biocontrol of pathogens. The objective of this study was to evaluate the biocontrol performances and the ability to induce a systemic response to crown gall of four strains belonging to Bacillus and Pseudomonas genera. Evaluation of in vitro antagonistic ability showed that the four bacteria had a different spectrum of activity against the three tested strains of Agrobacterium tumefaciens. A delay in time of appearance and a significant reduction of the tumor size were observed in tomato plants obtained by bio-primed seeds and stem inoculated with A. tumefaciens. Seed priming with antagonistic strains stimulated some systemic defense mechanisms in tomato plants that can be related to the reduction of disease symptoms induced by A. tumefaciens. Both antagonists and A. tumefaciens strains induced variations in phenol content and peroxidase activity while polyphenol oxidase activity was mainly affected by the single A. tumefaciens strain. The response was different in relation to the various combinations of antagonist/pathogen, however Pseudomonas brassicacearum EPR3 strain was the most effective strain.





Similar content being viewed by others
References
Aksoy, H. M., Kaya, Y., Ozturk, M., Secgin, Z., Onder, H., & Okumus, A. (2017). Pseudomonas putida-induced response in phenolic profile of tomato seedlings (Solanum lycopersicum L.,) infected by Clavibacter michiganensis subsp. michiganensis. Biological Control, 105, 6–12.
Altin, N., Bora, T. (2001). Biological control studies by Fluorescent Pseudomonads against to Erwinia carotovora subsp. carotovora Jones. Bergey et al. Caused Soft Rot on Potato. Ninth Turkish Phytopathology Congress, 3–8 Sept 2001, Tekirdag, Turkey, pp. 104–110.
Babu, A. N., Jogaiah, S., Ito, S., Nagaraj, A. K., & Tran, L. P. (2015). Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Science, 231, 62–73.
Backert, S., & Meyer, T. F. (2006). Type IV secretion systems and their effectors in bacterial pathogenesis. Current Opinion in Microbiology, 9, 207–217.
Beckers, G. J. M., & Conrath, U. (2007). Priming for stress resistance: From the lab to the field. Current Opinion in Plant Biology, 10, 425–431.
Ben Abdallah, D., Frikha-Gargouri, O., & Tounsi, S. (2015). Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. Journal of Applied Microbiology, 119, 196–207.
Caihong, H., Qian, Y. (2007). Advances in biocontrol mechanism and application of Trichoderma spp. for plant diseases. Journal of Northeast Agricultural University, v .14, p.161–167.
Dandurishvili, N., Toklikishvili, N., Ovadis, M., Eliashvili, P., Giorgobiani, N., et al. (2010). Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumors on tomato plants. Journal of Applied Microbiology., 110, 341–351.
Hammerschmidt, R., Nuckles, E. M., & Kuc, J. (1982). Association of enhanced peroxidase activity with induced systemic resistance of cucumber of Colletotrichum lagenarium. Physiological Plant Pathology, 20, 73–82.
Hao, F., Wang, L., Cao, K., Wang, X., Fang, W., Zhu, G., & Chen, C. (2015). Systemic acquired resistance induced by Agrobacterium tumefaciens in peach and differential expression of PR1 genes. HortScience, 50, 666–672.
Kahla, Y., Zouari-Bouassida, K., Rezgui, F., Trigui, M. and Tounsi, S. (2017). Efficacy of Eucalyptus cinerea as a source of bioactive compounds for curative biocontrol of crown gall caused by Agrobacterium tumefaciens strain B6. BioMed Research International, 10 p.
Kavino, M., Harish, S., Kumar, N., Saravanakumar, D., Damodaran, T., Soorianathasundaram, K., & Samiyappan, R. (2007). Rhizosphere and endophytic bacteria for induction of systemic resistance of banana plantlets against bunchy top virus. Soil Biology Biochemistry, 39, 1087–1098.
Kawaguchi, A. (2013). Biological control of crown gall on grapevine and root colonization by nonpathogenic Rhizobium vitis strain ARK-1. Microbes Environments., 28(3), 306–311.
Krimi, Z., Petit, A., Mougel, C., Dessaux, Y., & Nesme, X. (2002). Seasonal fluctuations and long-term persistence of pathogenic populations of Agrobacterium spp. in soils. Applied and Environmental Microbiology, 68, 3358–3365.
Krimi, Z., Raio, A., Petit, A., Nesme, X., & Dressaux, Y. (2006). Eucalyptus occidentalis plantlets are naturally infected by pathogenic Agrobacterium tumefaciens. European journal of plant pathology, 116, 237–246.
Krimi, Z., Alim, D., Djellout, H., Tafifet L., Mohamed mahmoud F. et Raio A. (2016). Bacterial endophytes of weeds are effective biocontrol agents of Agrobacterium spp., Pectobacterium spp. and promote growth of tomato plants. Phytopathologia mediterranea 55, 2, 184–196.
Maffei, M. E., Mithöfer, A., & Boland, W. (2007). Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry, 68(22), 2946–2959.
Magnin-Robert, M., Trotel-Aziz, P., Quantinet, D., Biagianti, S., & Aziz, A. (2007). Biological control of Botrytis cinerea by selected grapevine-associated bacteria and stimulation of chitinase and β-1,3 glucanase activities under field conditions. European Journal of Plant Pathology, 118, 43–57.
Mahmood, A., Turgay, O˘., Farooq, M., and Hayat, R. (2016). Seed biopriming with plant growth promoting rhizobacteria: a review. FEMS Microbiology Ecology, 92.
Mayer, A. M., Harel, E., & Shaul, R. B. (1965). Assay of catechol oxidase a critical comparison of methods. Phytochemistry, 5, 783–789.
Moore, L.W., Kado, C.I. and Bouzar, H. (1988). Agrobacterium. In Laboratory guide for identification of plant pathogenic bacteria. 2nded. New York, APS, Minnesota, USA. 158p.
Murthy, K. N., Uzma, F., Chitrashree, & Srinivas, C. (2014). Induction of systemic resistance in tomato against Ralstonia solanacearum by Pseudomonas fluorescens. American Journal of Plant Sciences., 5, 1799–1811.
Otten, L. Burr, T. Szegedi, E. (2008). Agrobacterium: A disease-causing bacterium. In: Tzfira T, Citovsky V, eds. Agrobacterium: From biology to biotechnology. New York, USA: Springer, 1–46.
Păcurar, D. I., Thordal-Christensen, H., Păcurar, M. L., Pamfil, D., Botez, C., and Bellini, C. (2011). Agrobacterium tumefaciens: From crown gall tumors to genetic transformation. Physiological and Molecular Plant Pathology . V 76, Issue 2, P 76–81.
Penyalver, R., and López, M. M. (1999). Co-colonization of the rhizosphere by pathogenic Agrobacterium strains and nonpathogenic strains K84 and K1026, used for crown gall biocontrol. 65:1936-1940.
Pieterse, C. M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C. M., & Bakker, P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology, 52, 347–375.
Pulawska, J. (2010). Crown gall of stone fruits and nuts, economic significance and diversity of its causal agents: Tumorigenic Agrobacterium spp. Journal Plant Pathology, 92, S87–S98.
Raio, A., Peluso, R., Puopolo, G., & Zoina, A. (2009). Evidence of pAgK84 transfer from Agrobacterium rhizogenes K84 to natural pathogenic Agrobacterium spp. In an Italian peach nursery. Plant pathology, 58, 754–753.
Rhouma, A., Ferchichi, A., Hafsa, M., & Boubaker, A. (2004). Efficacy of the non pathogenic Agrobacterium strains K84 and K1026 against crown gall in Tunisia. Phytopathologia Mediterranea, 43, 167–176.
Rhouma, A., Bouri, M., Boubaker, A., & Nesme, X. (2008). Potential effect of rhizobacteria in the management of crown gall disease caused by Agrobacterium tumefaciens biovar 1. Journal of Plant Pathology, 90, 517–526. https://doi.org/10.4454/jpp.v90i3.696.
Ryu, C. M., Murphy, J. F., Mysore, K. S., & Kloepper, J. W. (2004). Plant growth-promoting rhizobacterial systemically protect Arabidopsis thaliana against cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. The Plant Journal., 39, 381–392.
Saikia, R., Kumar, R., Arora, D. K., Gogoi, D. K., & Pzad, P. (2006). Pseudomonas aeruginosa inducing rice resistance against Rhizoctinia solani production of salicylic acid and peroxidase. Folia Microbiologica, 51(5), 375–380.
Seleim, M. A., Abo-Elyousr, K. A., Mohamed, A. A. A., & Al-Marzoky, H. A. (2014). Peroxidase and polyphenoloxidase activities as biochemical markers for biocontrol efficacy in the control of tomato bacterial wilt. Journal of Plant Physiology & Pathology., 2, 1.
Seskar, M., Shulaev, V., & Raskin, I. (1998). Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiology, 116(1), 387–392.
Singleton, V. L., Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture.16:144-58.
Smith, L., Keefe, D. O., Smith, M., & Hamill, S. (2003). The benefits of applying rhizobacteria to tissue cultured bananas. Banana Topics Newsletter, 33, 1–4.
Stonier, L. (1960). Agrobacterium tumefaciens (Conn II) – Production of an antibiotic substance. Journal of Bacteriology, 79, 880–898.
Zieslin, N., & Ben-Zaken, R. (1993). Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiology and Biochemistry, 31(3), 333–339.
Acknowledgements
The authors are grateful to Dr. Chemate Smail and Ms. Touati Souad for their valuable assistance in HPLC analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research does not involve any human participants and/or animals. The materials in the article have not been published in whole or in part elsewhere and not currently being considered for publication in another journal.
Conflict of interest
The authors declare that no known conflicts of interests exists.
Electronic supplementary material
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Djellout, H., Raio, A., Boutoumi, H. et al. Bacillus and Pseudomonas spp. strains induce a response in phenolic profile and enhance biosynthesis of antioxidant enzymes in Agrobacterium tumefaciens infected tomato plants. Eur J Plant Pathol 157, 269–280 (2020). https://doi.org/10.1007/s10658-020-01975-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-01975-1