Abstract
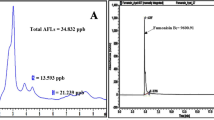
The main objective of this study was to investigate the antifungal activities of a culture filtrate from Streptomyces philanthi RL-1-178 (culture filtrate RL-1-178) on growth and aflatoxin B1 production of Aspergillus parasiticus TISTR 3276 and A. flavus PSRDC-4 inoculated onto maize seeds. The culture filtrate RL-1-178 was first analyzed for its composition and found to contain 86 compounds with acetic acid (53.59%) as the major component. It could inhibit both the growth and the production of aflatoxin B1 (AFB1) of the two aflatoxigenic species. Complete prevention of AFB1 production was achieved with 5% (v/v) of the culture filtrate RL-1-178. It also inhibited spore germination depending on the duration of treatment. The culture filtrate RL-1-178 altered on the cell wall composition of the two aflatoxigenic fungal strains by causing loss of cytoplasm and distortion of the plasma membranes evident by transmission electron microscopy (TEM) image. For testing on maize seeds, the culture filtrate RL-1-178 at 30% (v/w of maize seeds) exhibited 100% and 98% inhibition on AFB1 production by A. parasiticus TISTR 3276 and A. flavus PSRDC-4, respectively, without affecting maize seed emergence. The culture filtrate RL-1-178 possessed antifungal activity against the two aflatoxigenic fungal strains grown on maize seeds. The effective dose was 30% (v/w) with no effect on the maize emergence (100%). Our results suggest that the culture filtrate from S. philanthi RL-1-178 can be used as a natural product that controls the two aflatoxigenic fungi.


Similar content being viewed by others
References
Abd-Alla, M. A. (2005). Effect of acetic acid fumigation on common storage fungi of some medicinal and aromatic seeds. Egyptian Journal of Phytopathology, 33, 77–86.
Abdel-Rhman, M.M. (2015). Improvement the production of maize (Zea Mays L.) crop by using particle bombardment. International conference on biological, civil and environmental engineering (BCEE-2015) February 3–4 2015, Bali (Indonesia), pp 74–79.
Asis, R., Muller, V., Barrionuevo, D. L., Araujo, S. A., & Aldao, M. A. (2009). Analysis of protease activity in Aspergillus flavus and A. parasiticus on peanut seed infection and aflatoxin contamination. European Journal of Plant Pathology, 124, 391–403.
Boukaew, S., & Prasertsan, P. (2014a). Suppression of rice sheath blight disease using heat stable culture filtrate of Streptomyces philanthi RM-1-138. Crop Protection, 61, 1–10.
Boukaew, S., & Prasertsan, P. (2014b). Factors affecting antifungal activity of Streptomyces philanthi RM-1-138 against Rhizoctonia solani. World Journal of Microbiology and Biotechnology, 30, 323–329.
Boukaew, S., Chuenchit, S., & Petcharat, V. (2011). Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili. BioControl, 56, 365–347.
Boukaew, S., Petlamul, W., Suyotha, W., & Prasertsan, P. (2016). Simultaneous fermentative chitinase and β-1,3 glucanase production from Streptomyces philanthi RM-1-1-38 and their antifungal activity against rice sheath blight disease. BioTechnologia, 97, 271–284.
Boukaew, S., Prasertsan, P., Troulet, C., & Bardin, M. (2017). Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp. BioControl, 62, 793–803.
Bressan, W. (2003). Biological control of maize seed pathogenic fungi by use of actinomycetes. BioControl, 48, 233–240.
Buchanan, R. L., Edelson-Mammel, S. G., Boyd, G., & Marmer, B. S. (2004). Influence of acidulant identify on the effects of pH and acid resistance on the radiation resistance of Escherichia coli O157:H7. Food Microbiology, 21, 51–57.
Bullerman, L. B., & Bianchini, A. (2007). Stability of mycotoxins during foodprocessing. International Journal of Food Microbiology, 119, 140–146.
Chen, Y. Y., Chen, P. C., & Tsay, T. T. (2016). The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biological Control, 98, 34–42.
Demain, A. L. (2000). Small bugs, big business: The economic power of the microbe. Biotechnology Advances, 18, 499–514.
El-Abyad, M. S., EI-Abyad, M. A., EI-Shanshoury, A. R., & EI-Sabbagh, S. M. (1993). Towards the biological control of fungal and bacterial diseases of tomato using antagonistic Streptomyces spp. Plant and Soil, 149, 185–195.
Hwang, B. K., Lim, S. W., Kim, B. S., Lee, J. Y., & Moon, S. S. (2001). Isolation and in vivoand in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Applied and Environmental Microbiology, 67, 3739–3745.
Kang, Z. S., Huang, L. L., Krieg, U., Mauler-Machnik, A., & Buchenauer, H. (2001). Effects of tebuconazole on morphology, structure, cell wall components and trichothecene production of Fusarium culmorumin vitro. Pest Management Science, 57, 491–500.
Kaur, T., Jasrotia, S., Ohri, P., & Manhas, R. K. (2016). Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiological Research, 192, 247–252.
Kim, H., Rim, S. O., & Bae, H. (2019). Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens. Biological Control, 128, 24–30.
Komala, V. V., Ratnavathi, C. V., Vijay Kumar, B. S., & Das, I. K. (2012). Inhibition of aflatoxin B1 production by an antifungal component, eugenol in stored sorghum grains. Food Control, 26, 139–146.
Lal, R. (2005). World crop residues production and implications of its use as a biofuel. Environment International, 31, 575–584.
Leelasuphakul, W., Sivanunsakul, P., & Phongpaichit, S. (2006). Purification, characterization and synergistic activity of β-1, 3-glucanase and antibiotic extract from an antagonistic Bacillus subtilis NSRS 89-24 against rice blast and sheath blight. Enzyme and Microbial Technology, 38, 990–997.
Li, Q., Jiang, Y., Ning, P., Zheng, L., Huang, J., Li, G., Jiang, D., & Hsiang, T. (2011). Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biological Control, 58, 139–148.
Li, W. R., Shi, Q. S., Ouyang, Y. S., Chen, Y. B., & Duan, S. S. (2013). Antifungal effects of citronella oil against Aspergillus niger ATCC 16404. Applied Microbiology and Biotechnology, 97, 7483–7492.
Minuto, A., Spadaro, D., Garibaldi, A., & Gullino, M. L. (2006). Control of soil borne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Protection, 25, 468–475.
Morsy, A. A., Abd-El-Kareem, F., & Abd-Alla, M. A. (2000). Effect of acetic acid fumigation on common storage fungi of some grains. Egyptian Journal of Phytopathology, 28, 95–106.
Ng'ang'a, J., Mutungi, C., Imathiu, S., & Affognon, H. (2016). Effect of triple-layer hermetic bagging on mould infection and aflatoxin contamination of maize during multi-month on-farm storage in Kenya. Journal of Stored Products Research, 69, 119–128.
Nogueira, J. H. C., Gonçalez, E., Galleti, S. R., Facanali, R., Marques, M. O. M., & Felício, J. D. (2010). Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. International Journal of Food Microbiology, 137, 55–60.
Pathuri, I. P., Reitberger, I. E., Huckelhoven, R., & Proels, R. K. (2011). Alcohol dehydrogenase 1 of barley modulates susceptibility to the parasitic fungus Blumeria graminis f.sp. hordei. Journal of Experimental Botany, 62, 3449–3457.
Plascencia-Jatomea, M., Viniegra, G., Olayo, R., Castillo-Ortega, M. M., & Shirai, K. (2003). Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromolecular Bioscience, 3, 582–586.
Pornpukdeewattana, S., Kerdpiboon, S., Jindaprasert, A., Pandee, P., Teerarak, M., & Krusong, W. (2017). Upland rice vinegar vapor inhibits spore germination, hyphal growth and aflatoxin formation in Aspergillus flavus on maize grains. Food Control, 71, 88–93.
Prapagdee, B., Kuekulvong, C., & Mongkolsuk, S. (2008). Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. International Journal of Biological Sciences, 4, 330–337.
Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G., & Steubaurt, W. (2003). Chitosan as antimicrobial agent:Applications and mode of action. Biomacromolecules, 4, 1457–1465.
Rabea, E. I., Badawy, M. E. I., Steurbaut, W., & Stevens, C. V. (2009). In vitro assessmentof N-(benzyl) chitosan derivatives against some plant pathogenic bacteria and fungi. European Polymer Journal, 45, 237–245.
Rammanee, K., & Hongpattarakere, T. (2011). Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food and Bioprocess Technology, 4, 1050–1059.
Reddy, K. R. N., Raghavender, C. R., Reddy, B. N., & Salleh, B. (2010). Biological control of Aspergillus flavus growth and subsequent aflatoxin B1 production in sorghum grains. African Journal of Biotechnology, 9, 4247–4250.
Reddy, K. R. N., Raghavender, C. R., Salleh, B., Reddy, C. S., & Reddy, B. N. (2011). Potential of aflatoxin B1 production by Aspergillus flavus strains on commercially important food grains. International Journal of Food Science and Technology, 46, 161–165.
Sakuda, S., Ono, M., Furihata, K., Nakayama, J., Suzuki, A., & Isogai, A. (1996). Aflastatin A, a novel inhibitor of aflatoxin production of Aspergillus parasiticus, from Streptomyces. Journal of the American Chemical Society, 118, 7855–7856.
Sakuda, S., Ono, M., Ikeda, H., Sakurada, M., Nakayama, J., Suzuki, A., & Isogai, A. (1999). Aflastatins: New Streptomyces metabolites that inhibit aflatoxin biosynthesis. In Biologically active natural products: Agrochemicals ed. cutler, H.G. and cutler, S.J (pp. 185–199). Boca Raton, FL: CRC Press.
Sakuda, S., Ono, M., Ikeda, H., Nakamura, T., Inagaki, Y., Kawachi, R., Nakayama, J., Suzuki, A., Isogai, A., & Nagasawa, H. (2000a). Blasticidin A as an inhibitor of aflatoxin production by Aspergillus parasiticus. Journal of Antibiotics (Tokyo), 53, 1265–1271.
Sakuda, S., Ikeda, H., Nakamura, T., Kawachi, R., Kondo, T., Ono, M., Sakurada, M., Inagaki, H., Ito, R., & Nagasawa, H. (2000b). Blasticidin A derivatives with highly specific inhibitory activity toward aflatoxin production in Aspergillus parasiticus. Journal of Antibiotics (Tokyo), 53, 1378–1384.
Sangmanee, P., & Hongpattarakere, T. (2014). Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control, 40, 224–233.
Sengun, I. Y., & Karapinar, M. (2004). Effectiveness of lemon juice, vinegar and their mixture in elimination of Salmonella typhimurium on carrots. International Journal of Food Microbiology, 96, 301–305.
Shaista, K., Memon, A. N., Ghanghro, A. B., & Ibtessam, T. (2011). Fractionation and characterization of seed storage proteins from different wheat varieties cultivated in Sindh on SDS-PAGE electrophoresis. Pakistan Journal of Nutrition, 10, 139–142.
Shakeel, Q., Lyu, A., Zhang, J., Wu, M., Chen, S., Chen, W., Li, G., & Yang, L. (2016). Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3-10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biological Control, 101, 59–68.
Sidhu, O. P., Chandra, H., & Behl, H. M. (2009). Occurrence of aflatoxins in mahua (Madhuca indica Gmel.) seeds: Synergistic effect of plant extracts on inhibition of Aspergillus flavus growth and aflatoxin production. Food and Chemical Toxicology, 47, 774–777.
Strohl, W. R. (2004). Antimicrobials. In A. T. Bull (Ed.), Microbial diversity and bioprocessing (pp. 336–355). Washington DC: American Society for Microbiology.
Tatsadjieu, N. L., Dongmo, P. M. J., Ngassoum, M. B., Etoa, F. X., & Mbofung, C. M. F. (2009). Investigations on the essential oil of Lippia rugosa from Cameroon for its potential use as antifungal agent against Aspergillus flavus link ex. fries. Food Control, 20, 161–166.
Tian, J., Zeng, X., Feng, Z., Miao, X., Peng, X., & Wang, Y. (2014). Zanthoxylum molle Rehd. Essential oil as a potential natural preservative in management of Aspergillus flavus. Industrial Crops and Products, 60, 151–159.
Tolouee, M., Alinezhad, S., Saberi, R., Eslamifar, A., Zad, S. J., Jaimand, K., Taeb, J., Rezaee, M. B., Kawachi, M., Shams-Ghahfarokhi, M., & Razzaghi-Abyaneh, M. (2010). Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. International Journal of Food Microbiology, 139, 127–133.
Toteja, G. S., Mukherjee, A., Diwakar, S., Singh, P., Saxena, B. N., Sinha, K. K., Sinha, A. K., Kumar, N., Nagaraja, K. V., Bai, G., Prasad, C. A., Vanchinathan, S., Roy, R., & Parkar, S. (2006). Aflatoxin B1 contamination in wheat grain samples collected from different geographical regions of India: A multicenter study. Journal of Food Protection, 69, 1463–1467.
Valdez-Ortiz, A., Medina-Godoy, S., Valverde, M. E., & Paredes-Lopez, O. (2007). A transgenic tropical maize line generated by the directtransformation of the embryo-scutellum byA. tumefaciens. Plant Cell Tiss Organ Cult, 91, 201–214.
VazJauri, P., Altier, N., & Kinkel, L. L. (2016). Streptomyces for sustainability. In S. Castro-Sowinski (Ed.), Microbial models: From environment to industrial sustainability, microorganism for sustainability 1 (pp. 251–276). Berlin: Springer.
Wen, Y., Hatabayashi, H., Arai, H., Kitamoto, H. K., & Yabe, K. (2005). Functionof the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Applied and Environmental Microbiology, 71, 3192–3198.
Yabe, K., Chihaya, N., Hamamatsu, S., Sakuno, E., Hamasaki, T., Nakajima, H., & Bennett, J. W. (2003). Enzymatic conversion of averufin to hydroxyversicolorone and elucidation of a novel metabolic grid involved in aflatoxin biosynthesis. Applied and Environmental Microbiology, 69, 66–73.
Yoshinari, T., Akiyama, T., Nakamura, K., Kondo, T., Takahashi, Y., Muraoka, Y., Nonomura, Y., Nagasawa, H., & Sakuda, S. (2007). Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology, 153, 2774–2780.
Zahed, N., Hosni, K., Brahim, N. B., Kallel, M., & Sebei, H. (2010). Allelopathic effect of Schinus molle essential oils on wheat germination. Acta Physiologiae Plantarum, 32, 1221–1227.
Zhang, F., Li, X. L., Zhu, S. J., Ojaghian, M. R., & Zhang, J. Z. (2018). Biocontrol potential of Paenibacillus polymyxa against Verticillium dahliae infecting cotton plants. Biological Control, 127, 70–77.
Zucchi, T. D., de Moraes, L. A. B., & de Melo, I. S. (2008). Streptomyces sp. ASBV-1 reduces aflatoxin accumulation by Aspergillus parasiticus in peanut grains. Journal of Applied Microbiology, 105, 2153–2160.
Acknowledgements
This research work was financially supported by the Agricultural Research Development Agency (Public Organization) (PRP5905021490) and Thailand Research Fund (RTA6080010). The copyediting service of RDO/PSU and proof-reading by Dr. Seppo Karrila are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare having no conflict of interest.
Human and animal studies
This research did not involve human and/or animal participants.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Boukaew, S., Petlamul, W. & Prasertsan, P. Efficacy of Streptomyces philanthi RL-1-178 culture filtrate against growth and aflatoxin B1 production by two aflatoxigenic fungi on maize seeds. Eur J Plant Pathol 156, 1041–1051 (2020). https://doi.org/10.1007/s10658-020-01955-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-01955-5