Abstract
Background
Toxigenic fungi (Aspergillus and Fusarium) and their metabolites represent the major cause of corn and corn-based products contamination and consequently lead to severe economic and health issues.
Aim
Our current study aimed to investigate the efficacy of using L. macroides Bac6 as a biological control agent against the toxigenic fungi; A. flavus f10 and F. proliferatum f30 and their mycotoxins.
Results
The results illustrated that A. flavus f10 produced the aflatoxins AFB1 and AFG2 with concentrations of 21.239 and 13.593 ppb, respectively. While F. proliferatum f30 produced fumonisin B1 (9600 ppb). Furthermore, L. macroides showed a high potential for inhibition of toxigenic fungal growth using a dual culture method. F. proliferatum f30 and A. flavus f10 were found to be inhibited by a percentage of 80 and 62.5%, respectively. The results were confirmed using the scanning electron microscope. The antagonistic bacteria, L. macroides, showed chitinase productivity and activity of 26.45 U/L and 0.12 U/mL/min, respectively, which illustrates its potential application as a biocontrol agent. The GC-MS analysis revealed an abundance of Pyrrolo[1,2-a] pyrazine-1,4-dione, Hexahydro in the bacterial supernatant that exhibited antifungal characteristics. L. macroides had a significant reduction of AFB1 and AFG2 produced by A. flavus f10, recording 99.25% and 99% inhibition, respectively. It also showed strong inhibition of fumonisin B1 (90% inhibition) produced by F. proliferatum f30. Conclusion: Thus, the current study is a prospective study evaluating for the first time the potential impact of L. macroides Bac6 against the toxigenic fungi and their toxins.
Similar content being viewed by others
Background
The world’s population is estimated to be 8 billion by 2025 and 9.8 billion by 2050 [1]. As a result, increasing global agricultural productivity is required to meet food demand [2, 3]. Grains, pulses, and oil seeds continue to play an important part in both human and animal nutrition around the world. Aspergillus flavus is typically found in decaying vegetation and soil [4]. This fungus is well-known for producing aflatoxin, a strong carcinogen that is dangerous to both people and animals [5, 6]. A. flavus may infect a diverse range of hosts, such as maize, cotton, and peanuts. [7]. Infections that develop during growth or storage might cause significant monetary losses [8]. Due to its capacity to create secondary metabolites such as aflatoxins, cyclopiazonic acid, and kojic acid, A. flavus is fatal [9]. In the host organism, these metabolites may result in cell damage and death [10]. A. flavus’s aflatoxins have the potential to harm people either immediately after exposure or over time, leading to conditions such as liver cancer, immune system suppression, and growth retardation [11, 12]. A filamentous fungus called Fusarium proliferatum infects maize and causes illnesses in plants. It creates mycotoxins, which, when consumed by people or animals eating tainted feed or crops, can have major negative effects on their health [13]. F. proliferatum infects maize plants, causing root, stalk, and ear rot as well as producing fumonisins, a mycotoxin that can taint maize kernels and pose a major risk to human and animal health, results in a serious health risk to consumers. [14]. Additionally, grains, including corn (maize), are susceptible to mycotoxins before and after harvest [15]. When specific fungal species are exposed to environmental conditions (e.g., water activity, temperature, pH, and intergranular gas composition), mycotoxins are produced. Mycotoxins from toxigenic fungal growth are produced in food and animal feeds via the secondary metabolism process [16]. Mycotoxins, in contrast to many bacterial toxins, are typically extremely stable when exposed to the conventional heating method used in the preparation of foods for human consumption [17]. These fungi and mycotoxins have serious consequences for human and animal growth and health [18,19,20]. Aflatoxin and fumonisin B1 are two mycotoxins that have been linked to cancer in humans and other mammals as well as renal and neurological disorders. [21, 22]. One of the primary issues in managing mycotoxigenic fungi and related mycotoxin, which is a source of contamination is that fungicides are ineffective at treating agricultural commodities, that are usually applied in the agricultural sector to treat diseases caused by fungi. [23, 24]. Biological control may be a long-term solution, safe for human and environmentally healthy, self-sustaining treatment strategy to manage mycotoxins, resulting in reduced agri-operational costs [25, 26]. Biological control is effective, and many new approaches are being developed, most of them are based on microbiological research and the usage of microbial organisms that inhibit fungal growth and detoxify mycotoxins [27]. Bio-acceptable approaches should not only be beneficial to the environment and the crop, but also to the producers and the consumers [27]. One of the approaches that are most important for ensuring humanity’s health and sustaining eco-friendly food production will unquestionably be the introduction and administration of biological and natural protective agents against fungal contamination. [28, 29]. Biocontrol methods include an antibiosis, mycoparasitism, competition, development of resistance in the host plant, and competition [30]. As a result, mycotoxigenic biocontrol by antagonistic agents is regarded as a promising strategy for minimizing and reducing toxin production by these fungal species, and thus reducing the hazards of these toxins on human health when consuming food products [31]. Flavobacterium aurantiacum B-184 had been effectively evaluated for aflatoxins destruction and was effective in eradicating Aspergillus toxins from liquids irreversibly [32, 33]. Lactobacillus plantarum CECT 749 CFS had a strong antifungal impact on maize kernels and maize ears against A. flavus and F. verticillioides, and FB1 and AFB1 levels were extremely dropped [34]. Bacillus velezensis RC 218 and Streptomyces albidoflavus RC 87B successfully reduced Fusarium Head Blight by up to 30%, its severity by up to 25%, and deoxynivalenol accumulation by up to 51% on durum wheat under field conditions [35, 36]. Interestingly, Lysinibacillus macroides, isolated from damaged Waste materials from fruits and vegetables were found to possess an inhibiting effect on food-borne microbes [37]. It has been proven that sugarcane bagasse can be applied for producing extracellular laccase on a large scale using Lysinibacillus macroides at a low cost and with ease [38]. Additionally, Lysinibacillus macroides produces some antimicrobial compounds and chitinase, glucanase, and protease, which are enzymes that have the ability to degrade the cell wall, are well-known to be used as biocontrol techniques. [39]. Lysinibacillus has the ability to down regulate the fungal-hyphal growth [40]. Additionally, by lowering the prevalence of Salmonella, which is well-known for producing significant morbidity in poultry, people, and other animals such as cattle and pigs, as well as in several plants, Lysinibacillus macroides was working as a natural control agent [41]. The most common way for these illnesses to spread is through the intake of contaminated food and drink [41]. It is proven that Lysinibacillus macroides shows a significant concentration reduction ability for chromium (VI), which is known as an accumulated pollutant in lakes in Mexico [42]. Lysinibacillus has been proven to be effective at pest management [43], improve crop production and clean up heavy metal-contaminated ecosystems [43]. Zinc-tolerant Lysinibacillus spp. are additionally known to boost maize production in Zinc-contaminated soil [43, 44].
All across the world, mycotoxins pose a serious threat to the safety of food. Farmers and consumers suffer financial losses in poor nations such as Egypt when food lots contain high toxin levels. Extremely polluted lots are either totally discarded or sold for an undesirable price. In years and locations with extremely high levels of mycotoxins, leading to significant food loss and waste. In low- and middle-income countries, where mycotoxin limits may not even exist or may not be routinely implemented, human health consequences can be substantially get worse [5]. For the bio-management of toxic fungus and their potential to manufacture mycotoxins, the use of bacterial strains is recommended as a non-chemical, successful, environmentally friendly, and affordable biological control strategy [45]. There are no published papers that demonstrate the potential effect of using L. macroides as a natural deterrent towards toxigenic fungi that are related to corn and its products; therefore, we aim for the first time to investigate the efficacy of L. macroides Bac6 as a natural barrier to toxigenic fungi (A. flavus f10 and F. proliferatum f30) and minimize their mycotoxins to reduce the loss of crops and increase the productivity and quality of the corn and its products, which will reflect on the consumers’ health.
Results
Molecular studies for the most predominant toxigenic fungi isolated from yellow-corn and cornflakes ( A. flavus f10 and F. proliferatum f30) strains
From the previous screening study performed by [46], A. flavus f10 and F. proliferatum f30 showed the highest productivity of mycotoxins, so they are representative producers of aflatoxins and fumonisin B1, respectively. A. flavus f10 and F. proliferatum f30 isolates were identified at the species level using macro- and microscopic characteristics. Moreover, A. flavus f10 and F. proliferatum f30 isolates were subjected to confirmation of their identification using molecular techniques. The molecular typing resulted in partial 18 S rRNA gene sequences of 582 and 521 bp for A. flavus f10 and F. proliferatum f30, respectively. The partial 18 S rRNA gene sequence of 582 bp of the representative A. flavus f10 isolate had a sequence with 99% similarity to Aspergillus flavus strain PUXX-FS06 (KR296888.1), Aspergillus flavus IFM 42,126 (LC602022.1), Aspergillus flavus strain TN533D12 (MH271095) and Aspergillus flavus strain USMG09 (KF434090.1) available in the GenBank database (S 1 A). The candidate isolate was identified as Aspergillus flavus f10, which belongs to the family Aspergillaceae, order Eurotiales, class Eurotiomycetes. Similarly, the partial 18 S rRNA gene sequence of 521 bp of F. proliferatum f30 isolate had a sequence with 100% similarity to Fusarium proliferatum strain HC01-1 (MT560215.1), Fusarium proliferatum strain TH12-5 (MT560218.1) and Fusarium proliferatum strain CF2 (MN658457.1), which are available in the Genbank database (S 1B). The chosen isolate was identified as Fusarium proliferatum, which belongs to the family Nectriaceae, order Hypocreales, class Sordariomycetes. The phylogenetic trees were built independently from various sequence alignments of 18 S rRNA genetic sequences. The obtained sequences of toxigenic fungal strains A. flavus f10 and F. proliferatum f30 were deposited in the genebank database under accession numbers OQ087136 and OQ087105, respectively.
Quantification of mycotoxins production by A. flavus f10 and F. proliferatum f30 strains using the HPLC technique
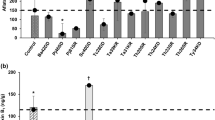
High-performance liquid chromatography analysis was employed to figure out the concentrations of aflatoxins and fumonisin B1 produced by A. flavus f10 and F. proliferatum f30 strains, respectively. HPLC analyses continued for 15–25 min of retention time, but all the produced mycotoxins were recovered at the first 6 min of retention time (Fig. 1) and the tested fungal strains showed a high ability to produce mycotoxins. A. flavus f10 strain showed the ability to produce two types of aflatoxins namely, AFB1 and AFG2 in considerable concentrations of 21.239 ppb and 13.593 ppb, respectively. Additionally, F. proliferatum f30 strain showed a high degree of toxigenicity since it produces 9600 ppb of fumonisin B1 (Fig. 2).
The antagonistic effect of bacterial isolates against the toxigenic A. flavus f10 and F. proliferatum f30 strains
Four bacterial isolates demonstrated various antagonistic activities against the two highest mycotoxin-producing fungal strains, A. flavus f10 and F. proliferatum f30. Bacterial isolate Lysinibacillus macrolides Bac6 exhibited the highest efficiency for inhibition of the growth of toxigenic fungi A. flavus f10 and F. proliferatum f30 recording a 13 mm and 8 mm inhibition zone, respectively. Whereas, bacterial isolates Bacillus subtilis, Pseudomonas sp. and Bacillus cereus showed inhibition zone of 8 mm, 4.5 mm and 2 mm, respectively against (A) flavus f10, as well as inhibition zone of 0.9 mm, 4.5 mm and 3 mm, respectively, against F. proliferatum f30 as shown in Table 1; Fig. 3A. Additionally, Lysinibacillus macroides Bac6 due to its highest efficacy among the four isolates as demonstrated in Fig. 3B. The accumulated data from three plates for each fungal strain are expressed as the mean ± SEM in L. macroides Bac6 (open bars), Bacillus subtilis (hatched bars), Pseudomonas sp. (herringbone bars) and Bacillus cereus (stripe bars). *P < 0.001, L. mocrides Bac6 vs. (B) subtilis; #P < 0.001, L. mocrides Bac6 vs. Pseudomonas sp.; and ; +P < 0.001, L. mocrides Bac6 vs. B. cereus.
Antagonistic activities of bacterial isolates against toxigenic fungal isolates A. flavus f10, and F. proliferatum f30 recovered from corn and corn-based products. (A)Lysinibacillus macroides Bac6, (B)Bacillus subtilis, (C)Pseudomonas sp. and (D)Bacillus cereus. The sum of the data from three plates for each fungal strain are expressed as the mean ± SEM in L. macroides Bac6 (open bars), Bacillus subtilis (hatched bars), Pseudomonas sp. (herringbone bars) and Bacillus cereus (stripe bars). *P < 0.001, L. mocrides Bac6 vs. B. subtilis; #P < 0.001, L. mocrides Bac6 vs. Pseudomonas sp.; and ; +P < 0.001, L. mocrides Bac6 vs. B. cereus
Reduction of toxigenic fungal growth by Lysinibacillus macroides Bac6 strain
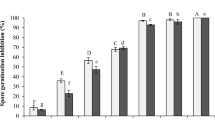
The confirmation of the reduction of toxigenic fungal growth with the most active bacterial strain Lysinibacillus macroides Bac6 was assayed on a solid medium using a dual culture method. The highest antagonistic bacterial strains exhibiting inhibition percentage (%) of fungal growth compared to control fungi were 80 and 62.5% for A. flavus f10, and F. proliferatum f30, respectively (Fig. 4). Interestingly, the bacterial strain L. macrolides Bac6 is the most potent antagonistic bacteria against the tested mycotoxigenic-producing fungal strains A. flavus f10 and F. proliferatum f30. Therefore, it was selected for further studies to illustrate its crucial role in minimizing the toxins produced by toxigenic fungi.
Spectrum of antifungal activities of L. macroides Bac6 against toxigenic A. flavus f10 and F. proliferatum f30 strains. The fungi in the first row (A) are the control (cultured fungi without bacterial effect), while those in the second row; (B) revealing substantial impacts on the development of the fungus in a dual culture after three days of incubation
Using morphological, microscopic, biochemical, and molecular methods, L. macroides Bac6 was identified. The partial 16 S rRNA gene sequence resulted in 931 bp; It was compared using a BLAST search (NCBI) with complete sequences that were available in the GenBank database. Sequences obtained with those retrieved from the GenBank database were subjected to Crustal analysis using Mega Align (DNA Star) for the phylogenetic analysis. Sequenced data were inserted in GenBank, and the resulting 16 S rRNA gene sequence showed identity similarity at 100% with L. macroides AzoM1 (MK942418.1), L. macroides JB_2 (MT197307.1) and L. macroides SMV311 (MN538917.1) (S2). Therefore, from the phylogenetic analyses, it can be identified as Lysinibacillus macroides Bac6 strain which, belongs to the family Bacillaceae, order Bacillales and class Firmicutes after which the resulting sequence was entered into the genebank with the corresponding accession number OQ080068.
Evaluation of the effect of Lysinibacillus macroides Bac6 strain on fungal growth of two toxigenic strains using electron microscopic examination
The impact of the highest antagonistic bacterial strain (Lysinibacillus macroides Bac6) on the growth of toxigenic fungi was evaluated using scanning electron microscope (SEM) to evaluate the possible mode of action of Lysinibacillus macroides Bac6 (Figs. 5B and 6B) against Aspergillus flavus f10 (Fig. 5A) and Fusarium proliferatum f30 (Fig. 6A). The resulting SEM graphs showed significant bacterial colonisation and persistent adhesion around the hyphae of toxigenic mould (Fig. 5C & 6C). The colonized hyphae displayed severely malformed and limited proliferation (Figs. 5D and 6D), pitting and damaged appearance of the hyphal cell wall compared with control fungal growth. As well as, it was noticed that, there is strong inhibition of conidia formation.
Scanning electron micrographs showed effect of Lysinibacillus macroides Bac6 on Aspergillus flavus f10 fungal growth. (A)Aspergillus flavus f10 growth “control”, (B)Lysinibacillus macroides Bac6 cells. (C, D) bacterial colonization around fungal hyphae, deformations, pitting and inhibition of sporulation
Scanning electron micrographs showed effect of Lysinibacillus macroides Bac6 on Fusarium proliferatum f30 fungal growth. (A)Fusarium proliferatum f30 growth “control”, (B)Lysinibacillus macroides Bac6 cells. (C, D) bacterial colonization around fungal hyphae, deformations, pitting and inhibition of sporulation
Chitinase activity
Scanning electron microscope (SEM) graphing showed pores formation of the toxigenic fungal hyphae when grown with L. macroides Bac6 strain in dual culture. Therefore, it was expected that the bacterial cells had the capability to produce hydrolyzing enzymes that induced fungal cell wall hydrolysis and pore formation by chitinase. The bacterial strain (L. macroides Bac6) was tested for chitinase enzyme production. Control without bacterial colony (Fig. 7A). It showed the capability to produce chitinase, recording the appearance of a clear zone of 12 mm after 24 h (Fig. 7B). Interestingly, it showed 55 mm inhibition zone on a solid chitin medium after 3 days incubation period (Fig. 7C). Moreover, using spectrophotometer, the highest antagonistic bacterial strain showed chitinase productivity of 26.45 U/L and chitinase activity of 0.12 U/mL/min. after 5 days incubation period.
Composition of the metabolites profiling of L. macroides Bac6
The bacterial metabolites analyses were performed using Gas Chromatography/Mass Spectrometry (GC-MS), to assay the bioactive compounds produced by the antagonistic bacterial strain that may be responsible for the inhibition of the growth of toxigenic fungi. The GC-MS analysis revealed that the most common bacterial metabolites were Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro (6.69%), 9,12,15-Octadecatrienoic acid,2,3-bis[(trimethylsilyl)oxy]propyl ester, (Z,Z,Z)- (6.57%), Hexadecenoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester (1.65%), Glycerol 2-acetate 1,3-dipalmitate (1.14), Ethyl iso-allocholate (1.07%). Additionally, 2(3 H)-furanone,5-heptyldihydro- (0.81%) of the total analytes. Whereas, the bacterial metabolites Agaricic acid (0.42%), Digotoxin (0.42%), N, N’-Bis (Carbobenzyloxy)-lysine methyl(ester) (0.33%), Oleic acid (0.29%), 2-Myristynoyl pantetheine (0.26%), 2(3 H)-Furanone, 5-heptyldihydro- (0.18%) and hexadecenoic acid methyl ester (0.15%) were detected in the GC-MS analysis (Fig. 8; Table 2).
Impact of L. macroides Bac6 strain on the formation of mycotoxins
The data from HPLC for each fungal extract with their toxins and the fungal extracts with their toxins treated by L. macroides are expressed as the concentration (ppb) in Table 3. *P < 0.0001, A. flavus f10 (AFB1) + L. macroides vs. A. flavus f10 (AFB1); # P < 0.0001, A. flavus f10 (AFG2) + L. macroides vs. A. flavus f10 (AFG2); and ; +P < 0.0001, F. proliferatum f30 (FB1) + L. macroides vs. F. proliferatum f30 (FB1). The resulted data revealed that the active bacterial cells had a significant reduction for aflatoxin B1 and aflatoxin G2 which were produced by Aspergillus flavus f10 recording 99.25% and 99% inhibition, respectively. In addition, the Lysinibacillus macroides showed strong inhibition of fumonisin B1 (90% inhibition) produced by Fusarium proliferatum f30 (Fig. 9).
Discussion
Multiple lines of evidence in vitro were employed to show that fumonisin and aflatoxins accumulations reflect the mycotoxigenic risk when the maize is infected with toxic Fusarium section Liseola and Aspergillus section Flavi [47]. Similarly, Izzati et al. [48] shown that F. proliferatum was found on nearly all maize farms. Other lines of evidence on corn were investigated and demonstrated the high occurrence of A. flavus [49, 50], as well as Fusarium species especially Fusarium graminearum and Fusarium proliferatum were very common in all maize-cultivated areas [51]. A. flavus, is the most risky worldwide species that is easily able to colonize corn [52]. Contamination with mycotoxins and their producers in maize and products based on corn is mainly caused by aflatoxins, fumonisins, and their primary fungal producers [53]. Regarding this F. proliferatum, F. verticillioides produced FB1 and FB2, with FB1 serving as the primary analogue (representing 75% of the total fumonisins). These isolates are thought to yield more than 500 g/g of FB1 [54]. Our results revealed that the potent toxigenic fungal strains A. flavus f10 and F. proliferatum f30 which are isolated from corn samples refered that strict quarantined and proper storage practices recommended to be used with imported goods in order to minimize infection with toxigenic moulds and steer clear of hazards to animal and human health.
The results were obtained from the HPLC method was employed to verify and quantify the aflatoxins (AFB1 – AFG2), and fumonisin B1 generated by the highest concentrations of active aflatoxins and strains that produce fumonisins, respectively, namely A. flavus f10, and F. proliferatum f30. Our data revealed that, Aspergillus flavus f10 that was invading cornflakes could produce aflatoxin B1 21.239 ppb and aflatoxin G2 13.493 ppb and F. proliferatum f30 produce 9600 ppb of fumonisin B1. Compared to another previous study that stated that A. flavus isolates could produce aflatoxin B1 in the range of 0.09–50.68 ppb and aflatoxin B2 0.33–9.2 ppb isolated from Malaysian Sweet Corn [55]. In other research, levels of Aflatoxins secreted by A. parasiticus and A. flavus isolated from silage of corn ranged 2–45 ppb and 2–100 ppb, respectively [56]. In addition, a study performed on corn and popcorn samples monitored that A. flavus var. columnaris and A. flavus isolates could produce aflatoxins with range of 1–8 ppb [57]. F. proliferatum and Fusarium verticillioides were investigated to accumulate FB1 and FB2 in maize kernels, which could result in a harmful or economical crop loss [58]. It is critical to find strategies to safeguard the maize crop from fumonisin building up. In the study performed [58], the production of fumonisin B1 is 6.22 ± 0.89 ppb. Similarly, Mohamed et al., published that FB1 was found in all silos samples and corn markets with a range of 13.69-175.54 ppb [59].
Our current study focused on the potentiality of using a biological control agent to reduce the growth of A. flavus f10 and F. proliferatum and their toxins. Our results revealed that L. macrolides Bac6 was the best one among all the tested bacterial isolates, due to its highest efficacy as demonstrated in Fig. 3B, which interpreted the data presented in Table 1. Although, there is no significance among the effects of the four tested bacterial isolates against both tested mycotoxigenic fungi. We demonstrated that the most antagonistic is L. macrolides Bac6, as a result of its safety as demonstrated in several research papers using it as a biological agent against pathogens [41, 60, 61]. Therefore, the Lysinibacillus macrolides Bac6 was the best choice to be used as a biological control agent. The proportion of fungal growth inhibited by Lysinibacillus macroides Bac6 as compared to control fungi was 80 and 62.5% for A. flavus f10 and F. proliferatum f30, respectively. As mentioned before in a previous study Bacillus megaterium BM344-1 has the ability to reduce the toxigenic fungi growth. The inhibition ratios (%) of P. verrucosum, (A) flavus, and F. verticillioides outperformed control fungi by 66.7, 29.4, and 18.2%, respectively [62]. Zeidan et al. [63] The most sensitive to yeast VOCs was found to be Penicillium followed by Aspergillus, whereas Fusarium was found to be the least sensitive. A key factor in fungus resistance is the nature of the fungal cell wall, which is affected by stressors in the microenvironment. The antagonistic effects of Bacillus volatiles as (B) subtilis, Bacillus amyloliquefaciens, Bacillus cereus, and B. megaterium towards toxigenic and phytopathogenic Penicillium and Aspergillus spp. have been illustrated [62, 64, 65].
We evaluated the underlying mechanism of the antagonistic effect of L. macrolides via electron microscopic examination, and we detected that the effect may be attributed to the production of chitinase enzyme. The tested Lysinibacillus macroides Bac6 strain in this study showed high potential production of exo-chitinase enzyme. According to the study that illustrated that Lysinibacillus spp. also produces some of these types of antimicrobial compounds, which include the development of cell wall-degrading enzymes as a biocontrol approach [40]. Cell wall-degrading enzyme-producer Lysinibacillus can inhibit fungal hyphal development [40].
Bacterial metabolites analysis performed by gas chromatography − mass spectrometry (GC − MS) revealed the presence of several compounds including Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro. This compound was the highest prevalent in the L. macroides Bac6 strain metabolites. Interestingly, it was reported as a bactericidal compound which also, isolated from Bacillus tequilensis MSI45 against multidrug-resistant pathogenic Staphylococcus aureus [66]. Moreover, it has been shown that Microcystis aeruginosa is susceptible to algicides, which is a compound was detected in Bacillus Lzh-5 [67]. Furthermore, GC-MS analysis of L. macroides Bac6 strain metabolites revealed that the presence of these bioactive compounds namely, Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro 2(3 H)-furanone,5-heptyldihydro, 9,12,15-octadecatrienoicacid,2,3 bis[trimethylsily] propylester, (Z, Z, Z)-, agaricic acid, digotoxin, ethyl iso-allocholate, oleic acid and hexadecanoic acid methyl ester. All compounds are well-known microbial biomolecules that have strong antagonistic activities against toxigenic as well as phytopathogenic fungi [68] [69] [70] [71]; [62, 72] [66]. While 9,12,15-octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (Z,Z,Z)- (6.57%) was the second molecule found with a large peak area and has been linked to antimicrobial activity [73], hexadecenoic acid has recently been linked to antifungal activity [74]. In addition, the 1-(hydroxymethyl)-1,2-ethanediyl ester (1.65%) had antifungal action [75], as did Glycerol 2-acetate 1,3-dipalmitate (1.14) [76], and Ethyl iso-allocholate (1.07%). Furthermore, 2(3 H)-furanone,5-heptyldihydro- (0.81% of total analytes) demonstrated antifungal activity. [77,78,79].
Aflatoxin and fumonisin-producing fungi share the same habitat as other microorganisms that can influence toxin production [80, 81]. Our results revealed that Lysinibacillus macroides Bac6 produced an inhibitory effect on A. flavus f10 and F. proliferatum f30 growth and their toxin production. Surprisingly, the Lysinibacillus macroides Bac6 metabolites inhibited AFB1 and AFG2 which produced by Aspergillus flavus f10, and FB1 that produced by Fusarium proliferatum f30, with 99.25, 99, and 90%, respectively. Saleh et al. [62] reported that aflatoxins (AFB1, AFG1, and AFG2), ochratoxin A, and FB1 production on artificial medium were completely inhibited after exposure to (A) flavus, P. verrucosum, and F. verticillioides to Bacillus megaterium BM344-1 VOCs. Moreover, Pereira et al. [81] study demonstrated that (B) amyloliquefaciens were significantly reducing FB1 and FB2 levels. Furthermore, it has been documented that volatiles released by B. megaterium KU143 and B. licheniformis 350-2 on un-hulled rice and maize ears prevent the production of aflatoxins by A. flavus [82], [83], respectively.
Conclusion
In conclusion, mycotoxins pose a serious threat to the safety of food, especially in low- and middle-income nations, incurring financial and human health consequences. Bacterial strains can be used as an eco-friendly, non-chemical, and low-cost biological control method. For the first time, we investigated the efficacy of using L. macroides Bac6 as a biological control agent against the most prevalent mycotoxigenic fungi invading corn and corn-based products through the potential impact of bacterial metabolites that significantly reduced AFB1 and AFG2 produced by A. flavus f10 and strongly inhibited fumonisin B1 produced by F. proliferatum f30. To better summarize, the methods and the resulted data of our study were demonstrated in (Fig. 10).
To summarize our study, we started with the isolation of the toxigenic fungi from corn samples and corn-based products, separation of the toxin after identification of the toxigenic fungi to be analyzed via HPLC to detect the types of mycotoxins, and finally illustrating the biological control effect of bacterial metabolites detected with GC-MS against those toxigenic fungi.
Materials and methods
Toxigenic fungi
Aspergillus flavus f10 and Fusarium proliferatum f30 strains were obtained from our previous work, in which they were isolated from yellow-corn and cornflakes, respectively [46]. According to our published paper, prior to usage, the tested fungal isolates were cultivated and cultured on potato dextrose agar (PDA) at 28 °C. [45, 84]. Both strains were identified by molecular characteristics based on 18 S rRNA.
Analyses of mycotoxins production by HPLC technique
The fungal inoculum preparation was carried out using freshly grown fungal cultures, seven days old, at 28 °C. A 1 cm disc was removed from the borders of the fungal growth using a cork-porer and utilized as fungal inoculum according to [85]. As an enhanced medium for mycotoxins formation, liquid yeast extract sucrose medium (YES) was employed containing (g/L); yeast extract, 1; peptone, 10; K2HPO4,1; MgSO4.7H2O, 0.5; sucrose, 30; KCl, 0.5; FeSO4.H2O, 0.01; and NaNO3, 2.0, and the pH was adjusted to 6.5. In 100 ml capacity conical flasks, 25 ml of the medium (YES) was pipetted, autoclaved, inoculated then incubated at 28 °C for two weeks and three weeks under static conditions for aflatoxins and fumonisin B1 production, respectively. For aflatoxins extraction, the homogenization occurred in the fungal broth. Using a fast speeds blender, mix 25 millilitres of chloroform solvent for a period of five minutes. The aqueous phase was subsequently filtered using Whatman filter paper No.1 after the organic phase was separated from it using a separating funnel, then dehydrated over a solution of anhydrous sodium sulphate, and dried to near dryness on a rotating evaporator. Each extract’s residuals were then reconstituted in two millilitres of chloroform then preserved in tiny brown bottles until the detection process. In contrast, the acetonitrile technique (5 ml/g of culture media) reported by [86] has been selected for fumonisin B1.
The mycotoxins were subjected to a quantitative estimation using high-performance liquid chromatography (HPLC) according to [87]. In the case of aflatoxins determination, the mobile phase comprised a combination of water, acetonitrile, and methanol (55:30:15 v/v/v), whereas, for fumonisin B1, two mobile phases have been used, namely solvent A (water: acetonitrile: acetic acid (59:4:1 v/v/v)) and solvent B acetonitrile: acetic acid (99:1 v/v). Aflatoxins and fumonisin B1 were detected using a detector that detects fluorescence with wavelengths of excitation of 295 and 335 nm, respectively, and emitting wavelengths of 330 and 440 nanometers, respectively [88,89,90]. The studies were carried out using HPLC system (Agilent Technologies Series 1200, G1321A FLD with column Zorbax, the Eclipse programme + C18) located at Assuit University’s Analytical Chemistry Unit.
Investigating the antagonistic activities of bacterial isolates against the toxigenic fungi
The antagonistic activities of the recovered bacterial isolates on the highest toxin-producing fungal strains (A. flavus F10 and F. proliferatum F30) were tested in vitro. The bacterial isolates recovered from soil were streaked with a line method at the center on PDA plates with a toxigenic strain of fungus inoculum, along with a 7-day incubation period at 28 °C for the cultures. Each treatment included three replications. The inhibitory zone was identified at the end of of the incubation time [91, 92].
Lysinibacillus macroides Bac6, the highest antagonistic bacteria, were selected for assay of the reduction of toxigenic fungal growth. Well-grown bacterial colonies which were incubated at 35 º C for 48 h were picked and further purified by streaking [93, 94]. The isolates were maintained on nutrient agar (NA) slants and stored at 4 ºC. The bacterial cultures were identified based on morphology (shape, Gram stain, spore formation and motility). L. macroides Bac6 has been identified by molecular characteristics based on 16 S rRNA [95].
Determination of the reduced level in toxic fungal growth by antagonistic bacteria Lysinibacillus macroides Bac6 strain
The decrease in the development of toxic fungi (A. flavus f10 and F. proliferatum f30) by Lysinibacillus macroides Bac6 strain was conducted according to the method described in [89, 92]. The interaction between the tested bacterial strains and toxigenic strains was assessed using PDA. 1 mL of bacterial suspension (107 CFU/mL) was mixed with 15 mL of molten PDA medium before solidification and poured into Petri dishes (90 mm diameter). After solidification of the medium, mycelial discs with 10 mm diameter were cut from the fungal active growing margins of 7 days old cultures and placed at the center of the agar surface. The control plates were inoculated with tested fungi but without bacteria following the same procedure. All plates were incubated at 28 °C for 72 h. The experiment was carried out in triplicates. Fungal growth inhibition was calculated by measuring the diameter of the fungal colonies using the following equation:
Where C is the diameter of the fungal colonies in control plates and.
T is the diameter of the fungal colonies in treated plates.
The alterations of fungal growth which was caused by antagonistic bacterial strains were shown and confirmed by scanning electron microscopy (SEM).
Evaluation of the impact of antagonistic bacterial strain on the Toxigenic fungal growth was evaluated by using scanning electron microscopy (SEM)
To evaluate the antagonistic capability of L. macroides Bac6 strain against the toxigenic fungi, the fungal mycelia of dual culture were examined using SEM. In brief, A 10-mm disc of mould growth boundary was removed and fixed for 2 days in 5% cool buffer glutaraldehyde. The samples were then rinsed three times (30 min each) with sodium cacodylate buffer before being post-fixed in 1% osmium tetroxide for two hours. The samples were then rinsed three times in the same buffer (30 min each) and dehydrated using an escalating ethanol gradient (30%, 50%, 70%, and 90%) for two hours and 100% ethanol for two days, before being treated with amyl acetate for a further two days. Following that, the samples were dried in a critical point drainer with liquid carbon dioxide before being attached to a metallic block with silver paint [91, 96].
Assay of chitinase activity of L. macroides Bac6 strain
Preparation of colloidal chitin
According to Hussin and Ab Majid, a colloidal of chitin was manufactured. Briefly, five grams powder of chitin were combined in a beaker with conc. Hydrochloric acid (~ 10 M HCl) 60 ml. Via a rod of glass, the prepared mixture was continuously stirred for a period of 5 min then 1 min gently stirring at a time interval 5 min for 1 h. In a 2 L conical flask, the chitin-HCl combination was subsequently processed using 2 L of cold distilled water and then it was kept under static conditions for 12 h at 4 ºC. The precipitation was gathered using crossing two distinct phases of until the colloidal chitin reached a pH of 7, the filter cloth was continuously rinsed with normal water. The produced colloid of chitin was squeezed between the filter paper (to reduce any residual moisture) and subsequently kept at 4 °C until it was used again [97].
Chitinase production of L. macroides Bac6 strain on solid medium
The initial screening was carried out by adding 10 L of a 24-hour-old culture of L. macroides Bac6 (107 CFU/mL) to the middle of NA plates that contained 1% colloidal chitin, then incubating those plates at 30 °C for 5 days. Congo red (1%) was used to demonstrate the activity of the enzyme for 30 s, after which the dye was fully removed with a solution of sodium chloride (30 g L-1) until a zone of transparency established as a result of chitin hydrolysis. The test was performed in three replicates. [98].
Chitinase enzyme activity of L. macroides Bac6 strain on liquid medium
A 250 mL Elementary flask with 100 mL NA and 1% colloidal chitin was filled with 10 mL of a 24-hour-old cultures of bacteria. The incubation was conducted on a rotating shaking device for five days at 30 °C and 180 rpm. The supernatant without cells was obtained by centrifuging the culture broths at a speed of 120 rpm for 10 min at 4 °C.
Applying the dinitrosalicylic acid (DNS) method, the efficacy of chitinase was assessed by looking for sugars that reduced after the enzymatic process. The test was performed with the specified methodology. [97]. After being produced in a 50 mM phosphate buffer (pH 7.0) with just one millilitre of crude extract of enzyme (cell-free culture supernatant), the substrate mixture consisted of 1% colloidal chitin. The resulting mixture had been warmed in a water bath at 37 °C for a period of one hour. By adding 3 mL of DNS reagent and boiling it in water for 20 min, the process was halted. The mixture was centrifuged at 5000 rpm for five minutes after cooling. The wavelength of the absorbance has been identified at 540 nm using the Model T60 UV-VIS spectrophotometer. The amount of chitinase activity (U/mL) was calculated as the amount of enzyme that, under normal test conditions, generated 1 mol of dinitrosalicylic acid per minute [99]. The experiment was carried out in triplicate.
Assessment of L. macroides Bac6 metabolites using GC-MS analyses
Tryptic soy broth (TSB) was used to culture the bacterial strain. The following ingredients were present in tryptic soy broth in grammes per litre: 17.0 for pancreatic digest of casein; 3.0 for papaic digest of soybean meal; sodium chloride, 5.0; dextrose, 2.5; and dibasic potassium phosphate, 2.5; pH after sterilisation was 7.30.2. Following a 24-hour incubation period at 30 °C, the bacterial culture was centrifuged for 5 min at 6000 g before being filtered through a sterile 0.22 micron membrane filter. With petroleum ether (1:1 v/v), the bacterial metabolites were separated from the resultant supernatant.
To quantify the bacterial metabolites, the GC/MS (Model: DPC-Direct Probe Controller (DPC20451), Thermo Scientific,USA; at the Chemistry Department, college of Science at Assiut University was used. A capillary column TG-5MS with dimensions of 30 m, 0.25 mm i.d., and 1 m film thicknesses was used for the separation of fatty acid ester compounds. The temperature of the oven was initially maintained at 80 °C for 5 min, and then increased at a ramp rate of 10 °C/min to 150 °C for 10 min, 200 °C at a ramping speed of 10 °C/min (held for 10 min), and 250 °C at a ramp rate of 5 °C/min (held for 13 min). Helium was employed as a carrying gas at a flow rate of 0.5 mL/min, and the split flow was 10 mL/min. [100, 101].
Effect of Lysinibacillus macroides Bac6 on mycotoxins production
Minimization of toxins production by toxigenic fungi by using active bacterial cells was tested by growing the fungus for 10 days in coculture with the bacterial living cells (107 CFU/ml), from 24 h old culture [90, 98]. As previously mentioned, high-performance liquid chromatography (HPLC) was used to extract the poisons and quantify their amounts.
Statistical analysis
GraphPad Prism software version 5 was used to conduct statistical analysis on normally distributed data, which are represented as means standard errors of the means (SEM). One-way ANOVA was used to analyse the significant differences between the three groups, and Tukey’s posttest followed.
Data Availability
All the generated data and the accession number for all the tested microorganisms are available and included in the manuscript.
References
Hoornweg D, Pope K. Population predictions for the world’s largest cities in the 21st century. Environ Urbanization. 2017;29(1):195–216.
Brock DA, Douglas TE, Queller DC, Strassmann JE. Primitive agriculture in a social amoeba. Nature. 2011;469(7330):393–6.
De Zeeuw H, Dubbeling M. Cities, food and agriculture: challenges and the way forward. Leusden: RUAF Foundation; 2009.
Griffin GJ, Garren KH. Colonization of rye green manure and peanut fruit debris by aspergillus falvus and aspergillus niger group in field soils. Appl Environ Microbiol. 1976;32(1):28–32.
Wu F. Mycotoxin risks are lower in biotech corn. Curr Opin Biotechnol. 2022;78:102792.
Tolosa J, Serrano Candelas E, Vallés Pardo JL, Goya A, Moncho S, Gozalbes R, et al. MicotoXilico: an interactive database to Predict Mutagenicity, Genotoxicity, and carcinogenicity of mycotoxins. Toxins. 2023;15(6):355.
Alam T, Anco DJ, Rustgi S. Management of Aflatoxins in Peanut. 2020.
Mesterházy Á, Oláh J, Popp J. Losses in the grain supply chain: causes and solutions. Sustainability. 2020;12(6):2342.
Zhi Q-Q, He L, Li J-Y, Li J, Wang Z-L, He G-Y, et al. The kinetochore protein spc105, a novel interaction partner of LaeA, regulates development and secondary metabolism in aspergillus flavus. Front Microbiol. 2019;10:1881.
Tebbi CK. Mycoviruses in Fungi: carcinogenesis of Fungal Agents May not always be Mycotoxin related. J Fungi. 2023;9(3):368.
Ensley SM, Radke SL. Mycotoxins in grains and feeds. Dis Swine. 2019:1055–71.
Tiwari S, Singh BK, Dubey NK. Aflatoxins in food systems: recent advances in toxicology, biosynthesis, regulation and mitigation through green nanoformulations. J Sci Food Agric. 2023;103(4):1621–30.
El-Sayed RA, Jebur AB, Kang W, El-Esawi MA, El-Demerdash FM. An overview on the major mycotoxins in food products: characteristics, toxicity, and analysis. J Future Foods. 2022;2(2):91–102.
Changwa R. Multi-mycotoxin contamination and associated dietary exposure in smallholder dairy farming systems of South Africa. University of Johannesburg (South Africa); 2021.
Del Fiore A, Reverberi M, Ricelli A, Pinzari F, Serranti S, Fabbri A, et al. Early detection of toxigenic fungi on maize by hyperspectral imaging analysis. Int J Food Microbiol. 2010;144(1):64–71.
Magan N, Aldred D. Why do fungi produce mycotoxins? Food Mycology. CRC Press; 2007. 135–48.
Awuchi CG, Ondari EN, Ogbonna CU, Upadhyay AK, Baran K, Okpala COR, et al. Mycotoxins affecting animals, foods, humans, and plants: types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods. 2021;10(6):1279.
Imran M, Cao S, Wan S, Chen Z, Saleemi MK, Wang N, et al. Mycotoxins–a global one health concern: a review. Agrobiological Records. 2020;2:1–16.
Saad-Hussein A, Ibrahim KS. Health Impact of Airborne Fungi. Handbook of Healthcare in the Arab World. 2021:1421-35.
Mahato DK, Devi S, Pandhi S, Sharma B, Maurya KK, Mishra S, et al. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: a review. Toxins. 2021;13(2):92.
Coppock RW, Dziwenka M. Chapter 32 - mycotoxins. In: Gupta RC, editor. Biomarkers in Toxicology. Boston: Academic Press; 2014. pp. 549–62.
Fletcher MT, Blaney BJ. Mycotoxins. Reference Module in Food Science. Elsevier; 2016.
Cleveland TE, Dowd PF, Desjardins AE, Bhatnagar D, Cotty PJ. United States Department of Agriculture—Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manage Science: Former Pesticide Sci. 2003;59(6–7):629–42.
Yazid SNE, Jinap S, Ismail SI, Magan N, Samsudin NIP. Phytopathogenic organisms and mycotoxigenic fungi: why do we control one and neglect the other? A biological control perspective in Malaysia. Compr Rev Food Sci Food Saf. 2020;19(2):643–69.
Fapohunda SO, Esan AO, Anjorin TS. Biological control of mycotoxins: an update. World’s Vet J. 2017;7:117–27.
Sahayaraj K. Basic and applied aspects of biopesticides. Springer; 2014.
Tsitsigiannis DI, Dimakopoulou M, Antoniou PP, Tjamos EC. Biological control strategies of mycotoxigenic fungi and associated mycotoxins in Mediterranean basin crops. Phytopathologia Mediterranea. 2012:158–74.
Behera BK, Varma A. Microbial biomass process technologies and management. Springer; 2017.
Habschied K, Kanižai Šaric G, Krstanovic V, Mastanjevic K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. In.: s Note: MDPI stays neutral with regard to jurisdictional claims in published ….
Legrand F, Picot A, Cobo-Díaz JF, Chen W, Le Floch G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol Control. 2017;113:26–38.
Sarrocco S, Mauro A, Battilani P. Use of competitive filamentous fungi as an alternative approach for mycotoxin risk reduction in staple cereals: state of art and future perspectives. Toxins. 2019;11(12):701.
Bata Á, Lásztity R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci Technol. 1999;10(6–7):223–8.
Umesha S, Manukumar HMg, Chandrasekhar B, Shivakumara P, Shiva Kumar J, Raghava S, et al. Aflatoxins and food pathogens: impact of biologically active aflatoxins and their control strategies. J Sci Food Agric. 2017;97(6):1698–707.
Nazareth TdM, Luz C, Torrijos R, Quiles JM, Luciano FB, Mañes J, et al. Potential application of lactic acid bacteria to reduce aflatoxin B1 and fumonisin B1 occurrence on corn kernels and corn ears. Toxins. 2019;12(1):21.
Palazzini J, Roncallo P, Cantoro R, Chiotta M, Yerkovich N, Palacios S, et al. Biocontrol of Fusarium graminearum sensu stricto, reduction of deoxynivalenol accumulation and phytohormone induction by two selected antagonists. Toxins. 2018;10(2):88.
Magan N, Aldred D, Mylona K, Lambert RJW. Limiting mycotoxins in stored wheat. Food Addit Contaminants: Part A. 2010;27(5):644–50. https://doi.org/10.1080/19440040903514523.
Ahmad V, Ahmad K, Baig MH, AL-Shwaiman HA, Al Khulaifi MM, Elgorban AM, et al. Efficacy of a novel bacteriocin isolated from Lysinibacillus sp. against Bacillus pumilus. LWT. 2019;102:260–7.
Abdelgalil S, Soliman N, Abo-Zaid G, Abdel-Fattah Y. Bioprocessing strategies for cost-effective large-scale production of bacterial laccase from Lysinibacillus macroides LSO using bio-waste. Int J Environ Sci Technol. 2021:1–20.
Coorevits A, Dinsdale AE, Heyrman J, Schumann P, Van Landschoot A, Logan NA, et al. Lysinibacillus macroides sp. nov., nom. Rev. Int J Syst Evol MicroBiol. 2012;62(Pt5):1121–7.
Ahsan N, Marian M, Suga H, Shimizu M. Lysinibacillus xylanilyticus strain GIC41 as a potential plant biostimulant. Microbes and Environments. 2021;36(4):ME21047.
Karmakar K, Bhattacharya R, Sharma A, Parmar K, Nath U, Nataraja KN, et al. Lysinibacillus macroides-mediated control of cellulose‐producing morphotype of Salmonella. J Sci Food Agric. 2022;102(14):6491–501.
Hernandez-Pena CC, Lares-Villa F, SANTOS-VILLALOBOS SDL, Estrada-Alvarado MI, Cruz-Soto A, Flores-Tavizon E, et al. Reduction in concentration of chromium (VI) by Lysinibacillus macroides isolated from sediments of the Chapala Lake, Mexico. Volume 93. Anais da Academia Brasileira de Ciências; 2021.
Ramli NA, Md Yusof NFF, Zarkasi KZ, Suroto A. Chemical, Biological and Morphological Properties of fine particles during local Rice Straw Burning Activities. Int J Environ Res Public Health. 2021;18(15):8192.
Jain D, Kour R, Bhojiya AA, Meena RH, Singh A, Mohanty SR, et al. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci Rep. 2020;10(1):1–13.
Ahmad Y, Ahmad MN, Zia A, Alam SS, Khan RAA, Riaz M. Biocontrol of economically important weed species through endophytic fungi isolated from Parthenium hysterophorus (family: Asteraceae). Egypt J Biol Pest Control. 2020;30(1):1–8.
Mohmoud ALE, Abdel-aziz AH, Hassan EA. ARTICLE INFO ABSTRACT.
Fandohan P, Hell K, Marasas W, Wingfield M. Infection of maize by Fusarium species and contamination with fumonisin in Africa. Afr J Biotechnol. 2003;2(12):570–9.
Izzati MNA, Azmi A, Nordahliawate MS, Norazlina J. Contribution to the knowledge of diversity of Fusarium associated with maize in Malaysia. Plant Prot Sci. 2011;47(1):20–4.
Agbetiameh D, Ortega-Beltran A, Awuah R, Atehnkeng J, Cotty P, Bandyopadhyay R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018;102(4):764–72.
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Allameh A, Kazeroon-Shiri A, Ranjbar-Bahadori S, Mirzahoseini H, et al. A survey on distribution of aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia. 2006;161(3):183–92.
Logrieco A, Mule G, Moretti A, Bottalico A. Toxigenic fusarium species and mycotoxins associated with maize ear rot in Europe. Mycotoxins in plant disease. Springer; 2002. 597–609.
Battilani P, Toscano P, der Fels-Klerx V, Moretti A, Camardo Leggieri M, Brera C, et al. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci Rep. 2016;6(1):1–7.
Yazid SNE, Ng WJ, Selamat J, Ismail SI, Samsudin NIP. Diversity and toxigenicity of mycobiota in Grain Corn: a Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia. Agriculture. 2021;11(3):237.
Proctor RH, Busman M, Seo J-A, Lee YW, Plattner RD. A fumonisin biosynthetic gene cluster in Fusarium oxysporum strain O-1890 and the genetic basis for B versus C fumonisin production. Fungal Genet Biol. 2008;45(6):1016–26.
Khan R, Ghazali FM, Mahyudin NA, Samsudin NIP. Chromatographic analysis of aflatoxigenic aspergillus flavus isolated from malaysian sweet corn. Separations. 2021;8(7):98.
Keller L, Pereyra MG, Keller K, Alonso VA, Oliveira A, Almeida T, et al. Fungal and mycotoxins contamination in corn silage: monitoring risk before and after fermentation. J Stored Prod Res. 2013;52:42–7.
Yassin MA, El-Samawaty A, Moslem M, Bahkali A, Abd-Elsalam K. Fungal biota and occurrence of aflatoxigenic aspergillus in postharvest corn grains. Fresenius Environ Bull. 2011;20(4):903–9.
Singh P, Dasgupta N, Singh V, Mishra NC, Singh H, Purohit SD, et al. Inhibitory effect of clove oil nanoemulsion on fumonisin isolated from maize kernels. LWT. 2020;134:110237.
Mohammed SW, Nayyef HJ, Sameer FO, Hanoon AY. Enzyme linked Immunosorbent Assay for Fumonisin B1 detection in local corn seeds from Baghdad-Iraq. Iraqi J Sci. 2021;62(12):4621–7.
Eski A, Demir I, Güllü M, Demirbağ Z. Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb Pathog. 2018;121:350–8.
Gautam P, Dubey SK. Biodegradation of Neonicotinoids: current Trends and Future prospects. Curr Pollution Rep. 2023:1–23.
Saleh AE, Ul-Hassan Z, Zeidan R, Al-Shamary N, Al-Yafei T, Alnaimi H, et al. Biocontrol activity of Bacillus megaterium BM344-1 against Toxigenic Fungi. ACS Omega. 2021;6(16):10984–90.
Alasmar R, Ul-Hassan Z, Zeidan R, Al-Thani R, Al-Shamary N, Alnaimi H, et al. Isolation of a novel Kluyveromyces marxianus strain QKM-4 and evidence of its volatilome production and binding potentialities in the biocontrol of toxigenic fungi and their mycotoxins. ACS Omega. 2020;5(28):17637–45.
Higazy NS, Saleh AE, Hassan ZU, Al Thani R, Migheli Q, Jaoua S. Investigation and application of Bacillus pumilus QBP344-3 in the control of aspergillus carbonarius and ochratoxin a contamination. Food Control. 2021;119:107464. https://doi.org/10.1016/j.foodcont.2020.107464.
Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, et al. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol. 2018;9:2363.
Kiran GS, Priyadharsini S, Sajayan A, Ravindran A, Selvin J. An antibiotic agent pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro isolated from a marine bacteria bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018;8(32):17837–46.
Li Z, Geng M, Yang H. Algicidal activity of Bacillus sp. Lzh-5 and its algicidal compounds against Microcystis aeruginosa. Appl Microbiol Biotechnol. 2015;99(2):981–90.
Teoh Y, Mashitah M, Salmiah U. Antifungal activities of selected wood-degrading fungi of rubberwood. J Trop for Sci. 2015:325–33.
Cantrell CL, Case BP, Mena EE, Kniffin TM, Duke SO, Wedge DE. Isolation and identification of antifungal fatty acids from the basidiomycete Gomphus floccosus. J Agric Food Chem. 2008;56(13):5062–8.
Salem MZM, Elansary HO, Elkelish AA, Zeidler A, Ali HM, Mervat E-H, et al. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. BioResources. 2016;11(4):9421–37.
de Rodríguez DJ, Trejo-González F, Rodríguez-García R, Díaz-Jimenez M, Sáenz-Galindo A, Hernández-Castillo F, et al. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. lycopersici. Ind Crops Prod. 2015;75:150–8.
Liu S, Ruan W, Li J, Xu H, Wang J, Gao Y, et al. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia. 2008;166(2):93–102.
Ibrahim OH, Mousa MA, Asiry KA, Alhakamy NA, Abo-Elyousr KA. Azadirachta indica A. Juss Fruit Mesocarp and Epicarp extracts induce Antimicrobial and Antiproliferative Effects against prostate (PC-3), breast (MCF-7), and colorectal adenocarcinoma (Caco-2) Cancer Cell Lines through Upregulation of proapoptotic genes. Plants. 2022;11(15):1990.
Prasath KG, Tharani H, Kumar MS, Pandian SK. Palmitic acid inhibits the virulence factors of Candida tropicalis: Biofilms, cell surface hydrophobicity, ergosterol biosynthesis, and enzymatic activity. Front Microbiol. 2020;11:864.
Azimkhanova BB, Ustenova GO, Sharipov KO, Rakhimov KD, Sayakova GM, Jumagaziyeva AB et al. Chemical Composition and Antimicrobial Activity of Subcritical CO2 Extract of Lepidium latifolium L.(Brassicaceae). International Journal of Biomaterials. 2021;2021.
Mohammed KA, Wasfy AA-HF, Bazalou MS. Comparison of Bioactive Components of Clove Buds as extracted by two different methods and analyzed by Gas Chromatography Triple Quad Time-Flight Technology.
Teoh YP, Don MM, Ujang S. Nutrient improvement using statistical optimization for growth of Schizophyllum commune, and its antifungal activity against wood degrading fungi of rubberwood. Biotechnol Prog. 2012;28(1):232–41.
Peng TY, Don MM. Antifungal activity of In-vitro grown Earliella Scabrosa, a malaysian fungus on selected Wood-degrading Fungi of rubberwood. J Phys Sci. 2013;24(2).
Sridharan A, Sugitha T, Karthikeyan G, Nakkeeran S, Sivakumar U. Metabolites of Trichoderma longibrachiatum EF5 inhibits soil borne pathogen, Macrophomina phaseolina by triggering amino sugar metabolism. Microb Pathog. 2021;150:104714.
Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181–200.
Pereira P, Nesci A, Castillo C, Etcheverry M. Field studies on the relationship between Fusarium verticillioides and maize (Zea mays L.): Effect of biocontrol agents on fungal infection and toxin content of grains at harvest. International Journal of Agronomy. 2011;2011.
Mannaa M, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against aspergillus and Penicillium spp. predominant in stored rice grains: study II. Mycobiology. 2018;46(1):52–63.
Hassan ZU, Al-Thani RF, Migheli Q, Jaoua S. Detection of toxigenic mycobiota and mycotoxins in cereal feed market. Food Control. 2018;84:389–94.
Filtenborg O, Frisvad JC, Thrane U. Moulds in food spoilage. Int J Food Microbiol. 1996;33(1):85–102.
Bagy MMK, Abd-Alla MH, Morsy FM, Hassan EA. Two stage biodiesel and hydrogen production from molasses by oleaginous fungi and Clostridium acetobutylicum ATCC 824. Int J Hydrog Energy. 2014;39(7):3185–97.
Nelson PE, Desjardins AE, Plattner RD. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry, and significance. 1993.
Sibanda L, De Saeger S, Van Peteghem C. Optimization of solid-phase clean-up prior to liquid chromatographic analysis of ochratoxin A in roasted coffee. J Chromatogr A. 2002;959(1–2):327–30.
Bailly JD, Querin A, Tardieu D, Guerre P. Production and purification of fumonisins from a highly toxigenic fusarium verticilloides strain. Revue de médecine vétérinaire. 2005;1(11):547–54.
Cubaiu L, Abbas H, Dobson AD, Budroni M, Migheli Q. A Saccharomyces cerevisiae wine strain inhibits growth and decreases ochratoxin a biosynthesis by aspergillus carbonarius and aspergillus ochraceus. Toxins. 2012;4(12):1468–81.
Algammal AM, Elsayed ME, Hashem HR, Ramadan H, Sheraba NS, El-Diasty EM, et al. Molecular and HPLC-based approaches for detection of aflatoxin B 1 and ochratoxin a released from toxigenic aspergillus species in processed meat. BMC Microbiol. 2021;21:1–10.
Nafady NA, Hashem M, Hassan EA, Ahmed HA, Alamri SA. The combined effect of arbuscular mycorrhizae and plant-growth-promoting yeast improves sunflower defense against Macrophomina phaseolina diseases. Biol Control. 2019;138:104049.
Zhan X, Khan RAA, Zhang J, Chen J, Yin Y, Tang Z, et al. Control of postharvest stem-end rot on mango by antifungal metabolites of Trichoderma pinnatum LS029-3. Sci Hort. 2023;310:111696.
Guo Y, Fan Z, Yi X, Zhang Y, Khan RAA, Zhou Z. Sustainable management of soil-borne bacterium Ralstonia solanacearum in Vitro and in vivo through fungal metabolites of different Trichoderma spp. Sustainability. 2021;13(3):1491.
Salah M, Badr G, Hetta HF, Khalifa WA, Shoreit AA. Fig latex inhibits the growth of pathogenic bacteria invading human diabetic wounds and accelerates wound closure in diabetic mice. Sci Rep. 2022;12(1):21852.
Badr G, El-Hossary F, Salah M, Khalaf M, Sayed EA, Elminshawy A. The therapeutic potential of cold atmospheric plasma against pathogenic bacteria inhabiting diabetic wounds. Bull Pharm Sci Assiut. 2023;–. https://doi.org/10.21608/bfsa.2023.216384.1770.
Yan L, Khan RAA. Biological control of bacterial wilt in tomato through the metabolites produced by the biocontrol fungus, Trichoderma harzianum. Egypt J Biol Pest Control. 2021;31(1):1–9.
Hussin NA, Ab Majid AH. Termiticidal activity of chitinase enzyme of Bacillus licheniformis, a symbiont isolated from the gut of Globitermes sulphureus worker. Biocatal Agric Biotechnol. 2020;24:101548.
Abdel-Kareem MM, Rasmey A, Zohri A. The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic aspergillus flavus. Lett Appl Microbiol. 2019;68(2):104–11.
Abirami S, Yogalsakshmi K, Pushpa ASR, Kananan M. Screening and identification of chitin degrading bacteria from shrimp shell waste dumping soil environment and its media optimization for chitinase enzyme production. World J Pharm Pharm Sci. 2016;5(11):743–57.
Hassan EA, Mostafa YS, Alamri S, Hashem M, Nafady NA. Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents. Plants. 2021;10(12):2737.
Yin N, Liu R, Zhao J-L, Khan RAA, Li Y, Ling J, et al. Volatile organic compounds of Bacillus cereus strain Bc-cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Dis. 2021;105(4):904–11.
Acknowledgements
Not applicable.
Funding
None.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.L.M., A.H.K., E.A.H. put the experimental design. A.L.M., A.H.K., E.A.H. performed all of the experiments.A.H.K., E.A.H. collected the food samples and investigated the fungal strains isolated from corn samples. A.L.M., A.H.K. performed all the experiments of mycotoxins detection and analysis. A.L.M., A.H.K., E.A.H. analyzed the data and drafted the manuscript; A.L.M., A.H.K., E.A.H. review and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study wasn’t require any ethical approval (Not applicable).
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmoud, A.E., Kilany, A.A. & Hassan, E.A. Antifungal activity of Lysinibacillus macroides against toxigenic Aspergillus flavus and Fusarium proliferatum and analysis of its mycotoxin minimization potential. BMC Microbiol 23, 269 (2023). https://doi.org/10.1186/s12866-023-03007-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03007-4