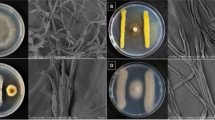
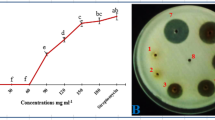
Abstract
The ability to control soil-borne pathogens in agriculture is highly conditioned by the restricted use of synthetic pesticides. Allelopathy, the antimicrobial activity of plant extracts, is a promising option against crop pathogens. Extracts from Lycium spp. such as L. barbarum, L. chinense and L. intricatum possess biological and therapeutic properties. Individual methanolic extracts from leaves and stems of the Mediterranean medicinal species L. europaeum collected in two locations of Tunisia were each evaluated in vitro against Verticillium dahliae (Vd), Sclerotinia sclerotiorum (Ss) and Harpophora maydis (Hm). The mycelial growth of the three fungi was significantly reduced by all the extracts at doses of 10 and 30 μl mL−1 (equivalent to 1 and 3 mg plant tissue mL−1). The sporulation of Hm was almost completely inhibited in all the amendments, but that of Vd was stimulated by one of the leaf extracts when 1 and 3 mg dried plant tissue mL−1 were used. Sclerotia of Ss were formed in a smaller number, their total weight increasing at extract doses equivalent to 1 mg plant tissue mL−1 and higher. In greenhouse, the pathogenicity of Hm was confirmed as early as 6 weeks after inoculation, since it caused significant decreases of weights in both roots and aboveground parts of maize. The detrimental effect of Hm on maize root weight in greenhouse was significantly counteracted by one of the leaf extracts added by watering. In total, 11 phenolic compounds were separated in the four extracts. The hydroxycinnamic acid family, including chlorogenic acid as a major compound, represented more than 50% of the total content in all the samples. Rutin was the most abundant flavonoid. The results of this work show the detrimental effect of L. europaeum extracts against the soil-borne pathogens Hm, Ss and Vd, and highlight their potential in crop protection if adequately developed into final products and used in combination with other tools.





Similar content being viewed by others
References
Abd El-Rahim, M. F., Fahmy, G. M., & Fahmy, Z. M. (1998). Alterations in transpiration and stem vascular tissues of two maize cultivars under conditions of water stress and late wilt disease. Plant Pathology, 47, 216–223.
Abdel-Monaim, M. F., Abo-Elyousr, K. A. M., & Morsy, K. M. (2011). Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Protection, 30, 185–191.
Abdennacer, B., Karim, M., Yassine, M., Nesrine, R., Mouna, D., & Mohamed, B. (2015). Determination of phytochemicals and antioxidant activity of methanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chemistry, 174, 577–584.
Abendroth, L. J., Elmore, R. W., Boyer, M. J., & Marlay, S. K. (2011). Corn growth and development, PMR 1009. Ames: Iowa State University Extension.
Agrios, G. N. (1970). Plant pathology. New York and London: Academic Press.
Alizadeh, H., Leung, D. W. M., & Cole, A. L. J. (2011). Conidiogenic effects of mannose-binding lectins isolated from cotyledons of red kidney bean (Phaseolus vulgaris) on Alternaria alternata. Phytochemistry, 72, 94–99.
Amagase, H., & Farnsworth, N. R. (2011). A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (goji). Food Research International, 44, 1702–1717.
Bergstrom, G., Leslie, J., Huber, D., Lipps, P., Warren, H., Esker, P., et al. (2008). Recovery plan for late wilt of corn caused by Harpophora maydis syn. Cephalosporium maydis. Washington, DC: National Plant Disease Recovery System.
Borrego-Benjumea, A., Basallote-Ureba, M. J., Melero-Vara, J. M., & Abbasi, P. A. (2013). Characterization of fusarium isolates from Asparagus fields in southwestern Ontario and influence of soil organic amendments on fusarium crown and root rot. Phytopathology, 104, 403–415.
Campbell, C. L., & Madden, L. V. (1990). Introduction to plant disease epidemiology. New York: John Wiley & Sons.
Carvalho, D. D. C., Alves, E., Batista, T. R. S., Camargos, R. B., & Lopes, E. A. G. L. (2008). Comparison of methodologies for conidia production by Alternaria alternata from citrus. Brazilian Journal of Microbiology, 39, 792–798.
Chellemi, D. O., Gamliel, A., Katan, J., & Subbarao, K. V. (2016). Development and deployment of systems-based approaches for the Management of Soilborne Plant Pathogens. Phytopathology, 106, 216–225.
Cheng, F., & Cheng, Z. (2015). Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Frontiers in Plant Science, 6, 1020. https://doi.org/10.3389/fpls.2015.01020.
Chou, C. H. (2010). Roles of allelopathy in plant biodiversity and sustainable agriculture. Critical Reviews in Plant Sciences, 18, 609–636.
Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12, 564–582.
Cushnie, T. P., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26, 343–356.
Degani, O., & Cernica, G. (2014). Diagnosis and control of Harpophora maydis, the cause of late wilt in maize. Advances in Microbiology, 4, 94–105.
El-Shafey, H. A., & Claflin, L. E. (1999). Late wilt. In D. G. White (Ed.), Compendium of corn diseases (3rd ed., pp. 43–44). St. Paul: American Phytopathological Society (APS Press).
Farooq, M., Jabran, K., Cheema, Z. A., Wahid, A., & Siddique, K. H. M. (2011). The role of allelopathy in agricultural pest management. Pest Management Science, 67, 493–506. https://doi.org/10.1002/ps.2091.
Fernández-Aparicio, M., Reboud, X., & Gibot-Leclerc, S. (2016). Broomrape weeds. Underground mechanisms of parasitism and associated strategies for their control: A review. Frontiers in Plant Science, 7, 135. https://doi.org/10.3389/fpls.2016.00135.
Fuentes-Alventosa, J. M., Rodríguez, G., Cermeño-Sacristán, P., Jiménez, A., Guillén, R., Fernández-Bolaños, J., et al. (2007). Identification of flavonoid diglycosides in several genotypes of asparagus from the Huétor Tájar population variety. Journal of Agricultural and Food Chemistry, 55, 10028–10035.
Gahukar, R. T. (2012). Evaluation of plant-derived products against pests and diseases of medicinal plants: A review. Crop Protection, 42, 202–209.
García-Ruiz, R., García-Carneros, A. B., & Molinero-Ruiz, L. (2014). A new race of Verticillium dahliae causing leaf mottle of sunflower in Europe. Plant Disease, 98, 1435. https://doi.org/10.1094/PDIS-04-14-0360-PDN.
Geiger, F., Bengtsson, J., Berendse, F., Weisser, W. W., Emmerson, M., Morales, M. B., Ceryngier, P., Liira, J., Tscharntke, T., Winqvist, C., Eggers, S., Bommarco, R., Pärt, T., Bretagnolle, V., Plantegenest, M., Clement, L. W., Dennis, C., Palmer, C., Oñate, J. J., Guerrero, I., Hawro, V., Aavik, T., Thies, C., Flohre, A., Hänke, S., Fischer, C., Goedhart, P. W., & Inchausti, P. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential of European farmland. Basic and Applied Ecology, 11, 97–105.
Ghali, W., Vaudry, D., Jouenne, T., & Marzouki, M. N. (2015). Lycium europaeum fruit extract: Antiproliferative activity on A549 human lung carcinoma cells and PC12 rat adrenal medulla Cancer cells and assessment of its cytotoxicity on cerebellum granule cells. Nutrition and Cancer, 67, 637–646. https://doi.org/10.1080/01635581.2015.1017054.
Grayer, R. J., & Harborne, J. B. (1994). A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry, 37, 19–42.
Gulya, T., Rashid, K., & Masirevic, S. (1997). Sunflower diseases. In A. A. Scheneiter (Ed.), Sunflower technology and production (pp. 263–379). Madison: Am. Soc. Agr., Crop Sci. Soc. Am. and Soil Sci. Soc. Am.
Heffer Link, V., & Johnson, K. B. (2007). White mold. The Plant Health Instructor. American Phytopathological Society. https://doi.org/10.1094/PHI-I-2007-0809-01.
Hernández-Castillo, F. D., Castillo-Reyes, F., Gallegos-Morales, G., Rodríguez-Herrera, R., & Aguilar-González, C. N. (2010). Lippia graveolens and Carya illinoensis organic extracts and their in vitro effect against Rhizoctonia solani Kühn. American Journal of Agricultural and Biological Sciences, 5, 380–384.
Hillocks, R. J. (2012). Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Protection, 31, 85–93.
Jasso de Rodríguez, D., Hernández-Castillo, D., Angulo-Sánchez, J. L., Rodríguez-García, R., Villarreal Quintanilla, J. A., & Lira-Saldivar, R. H. (2007). Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani, and Fusarium oxysporum. Industrial Crops and Products, 25, 111–116.
Jasso de Rodríguez, D., Trejo-González, F. A., Rodríguez-García, R., Díaz-Jimenez, M. L. V., Sáenz-Galindo, A., Hernández-Castillo, F. D., Villarreal-Quintanilla, J. A., & Peña-Ramos, F. M. (2015). Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. Sp. lycopersici. Industrial Crops and Products, 75, 150–158.
Kumar, S., & Pandey, A. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013, 1–16. https://doi.org/10.1155/2013/162750.
Langcake, P., Irvine, J. A., & Jeger, M. J. (1981). Alternative chemical agents for controlling plant disease. Philosophical Transactions of the Royal Society B, 295, 83–101.
Latha, P., Anand, T., Ragupathi, N., Prakasam, V., & Samiyappan, R. (2009). Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biological Control, 50, 85–93.
Lattanzio, V., Lattanzio, V. M. T., & Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In F. Imperato (Ed.), Phytochemistry: Advances in research (pp. 23–67). Kerala: Research Signpost.
Martínez, G., Regente, M., Jacobi, S., Del Rio, M., Pinedo, M., & de la Canal, L. (2017). Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pesticide Biochemistry and Physiology, 140, 30–35. https://doi.org/10.1016/j.pestbp.2017.05.012.
Martin-Sanz, A., Rueda, S., Garcia-Carneros, A. B., Gonzalez-Fernandez, S., Miranda-Fuentes, P., Castuera-Santacruz, S., et al. (2018). Genetics, host range, and molecular and pathogenic characterization of Verticillium dahliae from sunflower reveal two differentiated groups in Europe. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00288.
Mocan, A., Vlase, L., Vodnar, D. C., Bischin, C., Hanganu, D., Gheldiu, A. M., et al. (2014). Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense mill. leaves. Molecules, 19, 10056–10073. https://doi.org/10.3390/molecules190710056.
Molinero-Ruiz, M. L., Dominguez, J., & Melero-Vara, J. M. (2002). Races of isolates of Plasmopara halstedii from Spain and studies on their virulence. Plant Disease, 86, 736–740.
Molinero-Ruiz, M. L., Pérez-Vich, B., Pineda-Martos, R., & Melero-Vara, J. M. (2008). Indigenous highly virulent accessions of the sunflower root parasitic weed Orobanche cumana. Weed Research, 48, 169–178. https://doi.org/10.1111/j.1365-3180.2007.00611.x.
Molinero-Ruiz, M. L., García-Ruiz, R., Melero-Vara, J. M., & Dominguez, J. (2009). Orobanche cumana race F: Performance of resistant sunflower hybrids and aggressiveness of populations of the parasitic weed. Weed Research, 49, 469–478. https://doi.org/10.1111/j.1365-3180.2009.00708.x.
Molinero-Ruiz, M. L., Melero-Vara, J. M., & Mateos, A. (2010). Cephalosporium maydis, the cause of late wilt in maize, a pathogen new to Portugal and Spain. Plant Disease, 94, 379.
Molinero-Ruiz, L., Delavault, P., Pérez-Vich, B., Pacureanu-Joita, M., Bulos, M., Altieri, E., & Domínguez, J. (2015). History of the race structure of Orobanche cumana and the breeding of sunflower for resistance to the parasitic weed: A review. Spanish Journal of Agricultural Research, 13, e10R01. https://doi.org/10.5424/sjar/2015134-8080.
Ochoa-Fuentes, Y. M., Cerna-Chávez, E., Landeros-Flores, J., Hernández-Camacho, S., & Delgado-Ortiz, J. C. (2012). Evaluation in vitro of the anti-fungal activity of four methanol plant extracts for the control of three species of Fusarium spp. Φyton, 81, 69–73.
Ortiz-Bustos, C. M., García-Carneros, A. B., & Molinero-Ruiz, L. (2015). La marchitez tardía del maíz (Zea mays L.) causada por Cephalosporium maydis en la Península Ibérica, y otros hongos asociados. Summa Phytopathologica, 41, 107–114. https://doi.org/10.1590/0100-5405/1998.
Ortiz-Bustos, C. M., Testi, L., García-Carneros, A. B., & Molinero-Ruiz, L. (2016). Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. European Journal of Plant Pathology, 144, 383–397. https://doi.org/10.1007/s10658-015-0775-8.
Pane, C., Francese, G., Raimo, F., Mennella, G., & Zaccardelli, M. (2017). Activity of foliar extracts of cultivated eggplants against sclerotinia lettuce drop disease and their phytochemical profiles. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-016-1126-0.
Pegg, G. F., & Brady, B. L. (2002). Verticillium wilts. New York: CABI Publishing ISBN 0851995292.
Potterat, O. (2010). Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica, 76, 7–19.
Rosado-Alvarez, C., Molinero-Ruiz, L., Rodríguez-Arcos, R., & Basallote-Ureba, M. J. (2014). Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Scientia Horticulturae, 171, 51–57.
Santana-Gálvez, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017). Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules, 22, E358. https://doi.org/10.3390/molecules22030358.
Singh, G., Passsari, A. K., Leo, V. V., Mishra, V. K., Subbarayan, S., Singh, B. P., et al. (2016). Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Frontiers in Plant Science, 7, 407. https://doi.org/10.3389/fpls.2016.00407.
Soliman, F. H. S., & Sadek, S. E. (1998). Combining ability of new maize inbred lines and its utilization in the Egyptian hybrid program. Bulletin of the Faculty of Agriculture, Cairo University, 50, 1–20.
Švecová, E., Proietti, S., Caruso, C., Colla, G., & Crinò, P. (2013). Antifungal activity of Vitex agnus-castus extract against Pythium ultimum in tomato. Crop Protection, 43, 223–230.
Švecová, E., Colla, G., & Crinò, P. (2017). Antifungal activity of Boerhavia diffusa L. extract against Phytophthora spp. in tomato and pepper. European Journal of Plant Pathology, 148, 27–34. https://doi.org/10.1007/s10658-016-1065-9.
Thines, M., & Kamoun, S. (2010). Oomycete–plant coevolution: Recent advances and future prospects. Current Opinion in Plant Biology, 13, 427–433.
Treutter, D. (2006). Significance of flavonoids in plant resistance: A review. Environmental Chemistry Letters, 4, 147–157. https://doi.org/10.1007/s10311-006-0068-8.
Wu, H. S., Zhou, X. D., Shi, X., Liu, Y. D., Wang, M. Y., Shang, X. X., Gu, D. L., Wang, W. Z., & Wu, C. W. (2014). In vitro responses of Fusarium oxysporum f.Sp. niveum to phenolic acids in decaying watermelon tissues. Phytochemistry Letters, 8, 171–178.
Acknowledgements
The authors thank A.B. García-Carneros for excellent technical assistance.
Funding
This research was partially supported by grants AGL2010–17909 (Ministerio de Economía y Competitividad, Spain) and P12-AGR1281 (Andalusian Government, Spain) and the European Regional Development Fund (ERDF). The stay of R. Tej was granted by the Ministry of Higher Education and Scientific Research in Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each of the authors declare that the/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
We hereby state that our manuscript complies to the Ethical Rules applicable for the European Journal of Plant Pathology.
Electronic supplementary material
ESM 1
(PPTX 539 kb)
Rights and permissions
About this article
Cite this article
Tej, R., Rodríguez-Mallol, C., Rodríguez-Arcos, R. et al. Inhibitory effect of Lycium europaeum extracts on phytopathogenic soil-borne fungi and the reduction of late wilt in maize. Eur J Plant Pathol 152, 249–265 (2018). https://doi.org/10.1007/s10658-018-1469-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1469-9