Abstract
Late blight is a devastating potato disease caused by Phytophthora infestans. This organism can reproduce asexually and sexually between the strains of two mating types named A1 and A2. Mating type is an important strain characteristic affecting the pathogen’s population structure. We validate different PCR markers for P. infestans mating type determination by comparison of the results obtained with the markers (W16, S1, PHYB) with the pairing test results for 26 isolates collected worldwide and for a group of 146 Polish isolates. This study identifies an interesting feature of the isolates of genotype US-1. For all these A1 mating type isolates, the product specific for A2 isolates is amplified using the marker W16. Analysis of sequences of W16 PCR products indicates high similarity of the US-1 isolates with modern A2 mating type isolates. When US-1 isolates are excluded from analysis, 95 and 96% of isolates are correctly assigned by markers W16 and S1, respectively, when compared with the pairing test results. Marker PHYB produces 14% of discrepant results with the pairing test. Our results show that molecular markers can be useful tools for P. infestans mating type determination, but their application should be preceded by validation in each local population since their efficiency may vary depending on a pathogen’s genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora infestans (Mont.) de Bary, a member of the Oomycota, is one of the most important destroyers of potato (Solanum tuberosum) and tomato (S. lycopersicum) worldwide. This pathogen causes a disease named late blight and is able to completely devastate plants within a week. Therefore the average costs of yield losses and chemical protection against late blight are high and calculated at over 6 billion USD worldwide (Haverkort et al. 2008). P. infestans is a hemibiotrophic organism, which can reproduce asexually (forming sporangia). Sporangia are dispersed by the wind for long distances and that causes a rapid spread of the disease (Govers et al. 1997). Sexual reproduction is possible between two strains of different mating types of the pathogen designated A1 and A2. The heterothallic nature of P. infestans was discovered by Smoot et al. (1958) and Gallegly and Galindo (1958).
The Toluca Valley in Mexico is considered to be near the centre of origin of P. infestans. Populations in this region reproduce sexually and the genetic diversity is high (Niederhauser 1956). In the history of P. infestans, several migration events can be distinguished. A single clonal lineage, HERB-1, which caused the first epidemics of late blight, was introduced to Europe in the 1840s and in the early 1900s this strain was replaced by US-1 (Yoshida et al. 2013). In the beginning, the population of P. infestans in Europe consisted of the A1 mating type solely. Strains of A2 mating type were introduced along with potato seed tubers to Europe and other parts of the world: South America, North Africa and Asia (Fry et al. 2009; Yuen and Andersson 2013; Goss et al. 2014). The first report about appearance of the A2 mating type outside Mexico came from Switzerland in the early 1980s (Hohl and Iselin 1984). These new strains were rapidly distributed over Europe, leading to sexual reproduction of P. infestans in the continent (Drenth et al. 1995; Shaw et al. 1985; Sujkowski et al. 1994; Andersson et al. 1998). As a result of sexual recombination, the genetic variation in populations of P. infestans increases. New, recombined strains may adapt to environmental changes, resistant hosts or application of fungicides, and then spread. Li et al. (2017) showed that asexual clonal lineages of P. infestans, which are often triploid, in stress conditions can change to a diploid genome constitution. The triploid genotypes are more heterozygous than diploid and preserve more genetic differences. Recovery of the diploid state makes sexual reproduction possible and in consequence increases adaptability and plasticity of P. infestans strains. Ongoing numerous changes in P. infestans populations in the world have major implications for late blight and make it more difficult to control (Andersson et al. 2009; Fry et al. 2015; Govers et al. 1997; Smart and Fry 2001).
An important consequence of P. infestans sexual reproduction is the occurrence of oospores. The thick–walled and robust oospores can survive dry and cold conditions in the soil without a host for years. Turkensteen et al. (2000) demonstrated that in the Netherlands the oospores have been able to survive in the soil up to 4 years. Fernández-Pavía et al. (2004) have showed the survival of oospores for 2 years in dry weather conditions in Mexico. The oospores of P. infestans are considered an additional inoculum source. They can infect the newly planted tubers causing late blight epidemics earlier in the season than expected (Andersson et al. 2009; Fry et al. 2013; Mayton et al. 2000). The mating of P. infestans and formation of oospores are complicated. Ko (1978) proved that oospores are not always the result of crossfertilization. He showed that two isolates of opposing mating types separated by a semipermeable membrane were able to form oospores. Direct physical contact of isolates is not necessary and the substances secreted by them are sufficient to stimulate the production of oospores by selfing in the opposite type. These hormonal substances, termed α1 for the A1 strains and α2 for A2 strains, were isolated and characterized (Ojika et al. 2011; Qi et al. 2005; Yajima et al. 2008). Synthetic mating type hormones show the same biological activity as the natural hormones of P. infestans and induce the oospores production (Harutyunyan et al. 2008; Molli et al. 2012).
P. infestans is a model organism in the Oomycota group and many researchers monitored the populations of this organism in several countries in the world. Other examples of heterothallic Phytophthora species are P. ramorum, P. capsici and P. cinnamomi. For all these species the differences in distribution and frequency of mating types play important roles in epidemiology. In European populations of P. ramorum, only isolates with A1 mating type occur and there is no evidence for sexual reproduction, but in populations from North America, where the strains of two mating types coexist, sexual reproduction is possible in the life cycle of this pathogen. It is interesting that until the year 2012 no natural recombinants of P. ramorum have been observed (Grünwald et al. 2012). In contrast, among P. capsici populations from the USA and South Africa the occurrence of sexual reproduction and high levels of genetic diversity have been described (Dunn et al. 2010; Gobena et al. 2012; Meitz et al. 2010). Although in populations of P. capsici from Peru and in most provinces of China asexual clonal lineages dominate (Hu et al. 2013; Hurtado-Gonzales et al. 2008). According to Jung et al. (2013) isolates of A2 mating type of P. cinnamomi are generally more aggressive and invasive than the A1 ones. When they were introduced to Australia, southern Europe and the USA they caused symptoms of disease on many native species.
The earliest method of mating type determination of P. infestans isolates was a pairing test on Petri dishes. With the development of molecular biology, new techniques have become available. Molecular markers associated with mating type of P. infestans have been developed by Judelson et al. (1995), Judelson (1996), Kim and Lee (2002) and later by Kim et al. (2005) and Zhang et al. (2006). The PCR technique enables diagnosis of the mating type fast and efficiently. The pairing test, compared to PCR methods, is more time- and labour-consuming and requires more resources. PCR techniques have disadvantages too. If the marker is based on the presence/absence polymorphism of the single PCR product specific for one mating type, it is necessary to use an internal PCR control or more markers to exclude false-negative results. The linkages between markers and the mating type locus are difficult to estimate and may differ in different populations. Often, more than one method is used to ensure that the mating type is correctly determined and the conclusions about its proportion in the population are true.
The main objective of the present study was to validate different available markers for P. infestans mating type identification and to confront their results with the data obtained in the pairing test. We tested three molecular markers in two groups of isolates: i) 146 contemporary Polish isolates; and ii) 26 isolates from different locations in the world. An additional goal was to estimate the frequency of recombinants between the mating type locus and molecular markers in P. infestans isolates from Poland.
Materials and methods
Sampling, isolation of pure cultures and extraction of DNA of P. infestans isolates
Two groups of P. infestans isolates were studied. Polish (146) isolates collected in 2011 tested in this study were described earlier by Brylińska et al. (2016). The isolates were sampled from a total of 25 fields in three regions of Poland. Based on the results of polymorphism analysis in 14 SSR loci, 136 unique genotypes were detected within the 146 P. infestans isolates (Brylińska et al. 2016). The other group consisted of 26 diversified isolates from eight countries (Table 1). The strains were received from the sources listed in Table 1. Mating type of these isolates and genotype, if available, were determined before by other laboratories using the pairing test. US-1 genotype was defined using RG57 RFLP probe.
Pure cultures of P. infestans isolates and mycelia for DNA isolation were obtained as described before in Brylińska et al. (2016). Before extraction of DNA, samples were frozen in liquid nitrogen and freeze dried. The GenElute™ Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, Saint Louis, Missouri, USA) was used to isolate P. infestans genomic DNA, according to the manufacturer’s instructions. The DNA samples were stored at −20 °C.
Pairing test
The mating type of P. infestans samples was determined by crossing the isolates of unknown mating type with the standard isolate 7818 of A1 and isolate 90128 of A2 mating type (Table 1) on rye A agar media according to the method described by Zarzycka (2001). The fragment of agar (diameter 8 mm) containing mycelium of unknown isolate was placed on a Petri dish at a distance of 6 cm from a fragment of the same size of a standard isolate culture. For each isolate the test was prepared in two replicates with each mating type (2 of A1 and 2 of A2). Plates were incubated in the dark at 16 °C for about 10 days or until oospores were produced. The results were observed under a light microscope. The presence of oospores indicated that the tested isolate was of the opposite mating type to the standard P. infestans isolate.
W16 CAPS assay
To identify A1 and A2 mating types of P. infestans isolates, PCR primers W16–1 (5’-AACACGCACAAGGCATATAAATGTA-3′) and W16–2 (5’-GCGTAATGTAGCGTAA-CAGCTCTC-3′) were used according to Judelson et al. (1995). The PCR was conducted in a 20-μl reaction mixture consisting of 2 μl of 10 x buffer (Genoplast Chemicals, Rokocin, Poland), 6 μM MgCl2 (25 mM), 100 μM dNTPs (Sigma-Aldrich, Saint Louis, Missouri, USA), 0.4 μM of each primer, 1 U of Taq DNA polymerase (Genoplast Chemicals, Rokocin, Poland) and 10–50 ng of template DNA. Thermal cycling conditions of reactions were as follows: initial denaturation for 5 min at 94 °C; 30 cycles of denaturation for 1 min at 94 °C, primer annealing for 1 min at 53 °C, primer elongation for 1 min at 72 °C; and a final elongation for 10 min at 72 °C. The PCR products were digested for three hours at 37 °C with the restriction enzyme BsuRI (Fermentas, Thermo Fisher Scientific, Waltham, Massachusetts, USA) with recognition site 5’-GGCC-3′. The digestion mixture contained per sample: 7.7 μl of H2O, 2 μl of buffer Red (Fermentas, Thermo Fisher Scientific, Waltham, Massachusetts, USA), 0.3 μl of (10u/μL) restriction enzyme and 10 μl of PCR product.
S1 SCAR assay
To detect P. infestans isolates of A1 mating type, we used primer pair S1a (5′-AGGAT- TTCAACAA-3′) and S1b (5’-TGCTTCCTAAGG-3′) (Judelson 1996). Each PCR reaction mixture was the same as for marker W16. Thermal cycling parameters of PCR reactions were as follows: initial denaturation for 5 min at 94 °C; 35 cycles of denaturation for 1 min at 94 °C, primer annealing for 1 min at 50 °C, primer elongation for 1 min at 72 °C; and a final elongation for 4 min at 72 °C.
PHYB SCAR assay
A2 mating type of P. infestans isolates was diagnosed using primers PHYB-1 (5’-GATCGGATTAGTCAGACGAG-3′) and PHYB-2 (5’-GCGTCTGCAAGGCGCATTTT-3′) (Kim and Lee 2002). The PCR was performed in 25-μl reaction mixtures consisting of 2.5 μl of 10 x buffer with MgCl2 (Genoplast Chemicals, Rokocin, Poland), 25 μM dNTPs (Sigma-Aldrich, Saint Louis, Missouri, USA), 0.4 μM of each primer, 1 U of Taq DNA polymerase (Genoplast Chemicals, Rokocin, Poland) and 10–50 ng of template DNA. Amplification parameters were as follows: initial denaturation for 5 min at 95 °C; 35 cycles of denaturation for 1 min at 94 °C, primer annealing for 1 min at 62 °C, primer elongation for 1 min at 72 °C; and a final elongation for 7 min at 72 °C.
All products of PCR reactions for markers S1, PHYB and restriction products of marker W16 were resolved on 1.5% agarose gels containing ethidium bromide (1 mg mL−1) and visualized on a UV transilluminator.
Sequencing
For PCR product sequence analysis of marker W16 (Table 1), one P. andina isolate and 12 isolates of P. infestans of different mating type and genotype were chosen from different geographic regions. Each PCR reaction was conducted in 40 μl using the same conditions as described above. The W16 PCR products that were homogenous after initial amplification were purified with a Clean-Up Kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s instructions and sequenced. Non-homogeneous products were cloned before the sequencing into blunt pCRScript Amp SK cloning vector (Promega, Madison, Wisconsin, USA). After ligation, E. coli Top10 chemocompetent cells were transformed and spread on lysogeny broth (LB) plates containing 100 μg/ml ampicillin and X-gal (40 μg/ml) for blue-white selection. White colonies were picked up and bacteria were used as a source of plasmid template in colony PCR for positive clone screening. Transformants containing a cloned insert of interest were sequenced bidirectionally using universal M13F and M13R primers. Sequencing reactions were done using the BigDye Terminator v3.1 kit (Life Technologies Polska Ltd., Warsaw, Poland) and the sequencing products were resolved on a ABI3730XL genetic analyser in the Laboratory of DNA Sequencing and Oligonucleotide Synthesis (oligo.pl, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland). Two clones for each of four isolates of P. infestans were sequenced.
Data analysis
Programme ChromasLite 2 was used to evaluate the quality of obtained sequences. Then consensus sequences were prepared using MultAlin online software (Corpet 1988). The phylogenetic relationships were analysed by programme MEGA6 (Tamura et al. 2013) using the maximum likelihood (ML) method and bootstrap analysis with 1000 replications.
Results
Pairing test
Pairing tests confirmed the results obtained by other laboratories for the 26 control P. infestans isolates (Table 1). We identified 12 isolates of A1 mating type and 13 isolates of A2 mating type. As expected, the isolate of P. andina did not produce oospores after pairing with P. infestans. Among 146 Polish P. infestans isolates from the year 2011, 94 isolates were A1 mating type and 52 isolates were A2 mating type according to the pairing test. The results of this test were treated as a reference mating type to which the results of marker assessments were compared. For 126 (86%) Polish isolates from the tested group we observed consistent results for the pairing test and all PCR markers (Table 2).
W16 CAPS assay
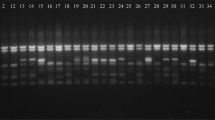
Primer pair W16–1 and W16–2 yielded a 557-bp product for all P. infestans isolates. After digestion with BsuRI endonuclease, three fragments (557 bp, 457 bp and 100 bp) were observed for the isolates of A1 mating type, while two fragments (457 bp and 100 bp) were obtained for P. infestans isolates of A2 mating type (Fig. 1a). Among 26 control isolates, according to the marker W16, 18 isolates were determined to be A2, seven were A1 and P. andina was A2 (Table 1). This group included seven A1 isolates which were wrongly assigned as A2 using the W16 marker: all five isolates of genotype US-1, the Mexican isolate TV580 and the isolate of P. andina. A Polish isolate (MP1358) was misidentified as A1 by this marker. According to the marker W16, the P. infestans isolates of A1 mating type (98) prevailed in the group of 146 Polish isolates (Table 2). Five A2 isolates in this group were classified as A1 contradictory to the pairing test, while one A1 isolate was misidentified as A2.
Agarose gels with PCR products amplified using the following primer sets: a – W16 (Judelson et al. 1995), products after restriction digestion by enzyme BsuRI, b – S1 (Judelson 1996) and c – PHYB (Kim and Lee 2002). The US-1 isolates of A1 mating type are shown in lanes: 1–9440507, 2–74001, 3–87000, 4–7818. The A2 mating type isolates are shown in lanes: 5–90128, 6–09-P15–07, 7–13/04/02, 8 – USD 618. The A1 mating type isolates not belonging to genotype US-1 are in lanes: 9–80029, 10 – EC-1, 11 – TV580. Line 12 contained products amplified from the P. andina isolate 3394. N– negative control without template DNA
S1 SCAR assay
After PCR reaction with S1a and S1b primers we observed a DNA fragment of approximately 1250 bp only for the isolates that were supposedly of A1 mating type (Fig. 1b). In the control group, 13 P. infestans isolates and P. andina showed the fragment specific for A1 mating type and for 12 isolates we did not observe any PCR product (Table 1). These results were in agreement with the pairing test results in the control group, except for isolate MP1358 from Poland, which was determined as A1. For two isolates (TV580 and P. andina) the results were in discordance with data for marker W16 among isolates from different countries. The data of mating type identification in the group of 146 Polish P. infestans isolates from the year 2011 for marker S1 corresponded well with the data for marker W16: only six isolates (4.1%) were determined incorrectly by markers W16 and S1 (Table 2) in comparison to the pairing test.
PHYB SCAR assay
Primer pair PHYB-1 and PHYB-2 was used to identify a 347-bp DNA fragment potentially specific for A2 mating type (Fig. 1c). According to this marker, 10 P. infestans isolates from the control group were designated as A2 mating type, while 15 isolates and P. andina were diagnosed as A1 mating type (Table 1). Isolate 05194 of A1 mating type, when tested with the PHYB marker, showed a PCR product characteristic of the A2 mating type. Four other isolates from the control group were misidentified as A1 (Table 1). Amongst 146 isolates of P. infestans from Poland, 45 isolates (31%) were determined by PHYB as of A2 mating type instead of 52 isolates identified as of A2 by the pairing test. For 15 isolates (10%) we observed differences between markers W16, S1 and PHYB in mating type determination (Table 2). The discrepancies between the results of the pairing test and marker PHYB reached 13% (19 isolates) in the group of 146 Polish P. infestans isolates.
Nucleotide sequences of marker W16
For sequencing of the PCR products obtained with marker W16, 12 P. infestans isolates: TV580 (A1), 80029 (A1), 88069 (A1), MP1444 (A1), 13/04/02 (A2), 88133 (A2), MP1358 (A2), MP1487 (A2), 07/39 (A2), IPO-0 (US-1, A1), 7818 (US-1, A1), 87,000 (US-1, A1) and one P. andina (A1) isolate, 3394, were chosen from the control group (Table 1). Among the chosen isolates there were some in which we observed discrepancies in mating type by different assessment methods and some for which all methods exactly corresponded. The sequences of the W16 marker obtained for 13 isolates were compared with the sequence of P. infestans reference isolate T30–4 (GenBank, AATU00000000.1, 1,677,613–1,678,169 bp). The W16 PCR products were sequenced with the forward and reverse primers directly after PCR and for 10 isolates we obtained sequences of good quality. For three isolates (MP1444, MP1358, 3394) we obtained illegible sequences due to heterogeneity of the products and for these isolates the W16 products were cloned and sequenced again. Thirteen SNPs, an insertion of 11 nucleotides in position from 519 to 529 bp and a deletion of an A stretch in position 203–208 bp were identified by using MultAlin software (Fig. 2). Among all tested sequences, we distinguished eight alleles (Fig. 2, Table 3). A Maximum Likelihood tree was constructed to illustrate the relationships among the alleles of P. infestans W16 sequences (Fig. 3). Three main clades were revealed. Clade 1 was the largest with alleles W8, W7, W6 and W5. Clade 2 was comprised of two alleles W1, W2 and this clade was closest to clade 1. The third clade consisted of alleles W4 and W3 and it was the most divergent from the others. Sequence analysis of PCR products showed that the isolates 07/39 (genotype 13_A2) and 13/04/02 of A2 mating type were homozygous and had W3 and W1 alleles, respectively. Isolates of A1 mating type that were not US-1 were heterozygous (C/T) in the recognition site of the restriction enzyme BsuRI located at position 101 bp (calculated from the first nucleotide of the primer) and had the following alleles: MP1444 – W7 and W8; MP1358 – W1 and W7; TV580 – W3 and W4; 80029 – W5 and W6 (Table 3). The isolates of genotype US-1 and P. andina, which were as well of A1 mating type, were homozygous like the isolates of A2 mating type in the recognition site of the restriction enzyme BsuRI. All US-1 isolates had allele W1. P. andina was heterozygous with alleles W1 and W2 (Table 3).
Incomplete alignment (only variable positions and restriction site are shown) of W16 sequences amplified from isolates of P. infestans from different origins. Reference genome sequence T30–4 (1677613–1678169 bp) was used to compare the tested fragments. Restriction site for BsuRI is in bold and “-“marks deletions
Discussion
The distribution and frequency of mating types and consequently the presence or absence of the sexual reproduction of P. infestans is widely investigated. Judelson et al. (1995) postulated that P. infestans mating type is determined by a single nuclear locus. They identified DNA markers linked to this locus using the RAPD (Random Amplified Polymorphic DNA) technique. Progeny from three crosses were used for analysis. A marker, W16 was mapped in that study with from 0.00 to 0.23 recombination fraction, depending on the cross (Judelson et al. 1995). Our results showed that, using the W16 marker, all isolates of P. infestans genotype US-1, isolate TV580 from Mexico, P. andina species and one isolate from a group of 146 Polish isolates from the year 2011 were incorrectly determined as A2 mating type in comparison to the pairing test. Additionally, five isolates from the year 2011 from Poland were wrongly designated as A1 by marker W16. The W16 product sequence analysis showed that it is a variable non-coding sequence. All tested isolates of genotype US-1 had allele W1, which is also present in most of the A2 mating type isolates except for isolate 07/39 (13_A2). In addition we did not observe, in the W16 marker sequences of isolates of genotype US-1 and A2 mating type, an insertion of 11 nucleotides, which was present in sequences of A1 mating type isolates that were not US-1. Furthermore, we observed homozygosity within US-1 and A2 P. infestans isolates for the BsuRI restriction site. Therefore, the marker W16 should not be applied in populations where isolates of genotype US-1 occur, because they are wrongly assigned as A2 mating type. Beketova et al. (2014) determined mating type among 796 P. infestans isolates from Russia by the pairing test and the W16 marker. The results of both tests matched in 90% of isolates. Among all of the 172 P. infestans isolates analysed in this study, only 5% were wrongly diagnosed by the marker W16. In contrast, in India, Chowdappa et al. (2015) used the pairing test and marker W16. All 157 tested isolates were of the A2 mating type and both mating type assessment methods were consistent. However, the 157 Indian isolates of P. infestans were all clones of the genotype 13_A2, therefore no conclusions on frequency of recombinants between marker and mating type locus can be drawn. Mazáková et al. (2010) did not detect any differences between the results of pairing tests and marker W16. The data obtained for the pairing test exactly corresponded with results of the PCR marker in the sample of 195 isolates from the Czech Republic. In that study, the isolates of both mating types were present.
The second marker developed by Judelson (1996) was SCAR marker S1. Locus S1 consisted of a large array of a tandemly repeated sequence detected in A1 mating type isolates. That author analysed progeny from seven crosses and an additional 95 P. infestans isolates from a worldwide collection. Only one of the 95 isolates was incorrectly classified by marker S1. Using the same marker, we observed one discrepancy (isolate MP1358) in the control group of P. infestans isolates that was classified wrongly as A1 in comparison to the pairing test. Among isolates from the year 2011, one was wrongly assigned as A2 and five were wrongly designated as A1 mating type. Among all P. infestans isolates we observed that 4% were wrongly diagnosed by the marker S1. This marker also was used by Mazáková et al. (2010) to determine mating type in isolates from the Czech Republic and all results were exactly matching the results of the pairing test.
The last marker validated in this research, marker PHYB associated with the A2 mating type, was developed using the Amplified Fragment Length Polymorphism (AFLP) technique (Kim and Lee 2002). This marker was shown to be specific for P. infestans since no products were observed for other Phytophthora spp. Kim and Lee (2002) tested presence of the expected PCR fragment only in a small group of 17 P. infestans isolates and the results were consistent with the pairing test but the tested group may have not been diverse enough to test reliably the marker’s linkage with the mating type locus. Within our results from the control group of P. infestans isolates, there were five isolates misidentified by marker PHYB. Moreover, 19 isolates were wrongly diagnosed in the group of Polish isolates from the year 2011 in comparison to the pairing test. Our results showed that the most discrepancies (14%) were observed for the marker PHYB. Chowdappa et al. (2015) did not detect any differences between the results of the pairing test and assessment by the PHYB marker in the group of 13_A2 isolates.
The 146 P. infestans isolates collected in Poland in 2011 were very diverse, as it was shown by analysis of neutral SSR loci, and they represented a population in which sexual recombination most likely occurs (Brylińska et al. 2016). The sexual recombination is also a likely reason of broken linkages between the markers and the mating type locus observed in six isolates (W16, S1) or even 19 isolates (PHYB).
Unambiguous identification of the mating type among P. infestans isolates is very important for population studies and has practical consequences for epidemiology and disease control. In Poland, where sexual reproduction most likely occurs, the ratio of A1 to A2 mating type changed in different years and depended on the region of collection. Sujkowski et al. (1994) tested 247 P. infestans isolates collected from year 1985 to year 1991. In the 1985–1987 period, the Polish isolates were representing a single clonal lineage of A1 mating type. After 1987 new genotypes were introduced and until year 1991 the population of P. infestans in Poland became more divergent with both mating types present. A further test of 93 Polish P. infestans isolates from 2002 to 2004 showed that the population consisted of different genotypes of two mating types, but with a predominance of A1 (Śliwka et al. 2006). Recently, in the Polish region Siedlce, Brylińska et al. (2016) observed a 1:1 proportion of A2 to A1 isolates. In regions Młochów and Boguchwała isolates of A1 mating type prevailed.
Application of molecular markers in determination of P. infestans mating type allows to test more isolates from a population, thanks to lowering cost as well as time and labour inputs. Bigger sample sets enable more reliable conclusions about the frequency of A1 to A2 mating type in the population by limiting the sampling effects. However, the results obtained by molecular markers are prone to mistakes resulting from recombination between the mating type locus and the marker. Our results show that molecular markers, W16 and S1, can be useful tools for P. infestans mating type determination but their application should be preceded by validation in each local population since their efficiency may vary depending on the pathogen’s genotype.
References
Andersson, B., Sandstrom, M., & Stromberg, A. (1998). Indications of soil borne inoculum of Phytophthora infestans. Potato Research, 41, 305–310.
Andersson, B., Widmark, A. K., Yuen, J. E., Nielsen, B., Ravnskov, S., Kessel, G. J. T., Evenhuis, A., Turkensteen, L. J., Hansen, J. G., Lehtinen, A., Hermansen, A., Brurberg, M. B., & Nordskog, B. (2009). The role of oospores in the epidemiology of potato late blight. Acta Horticulturae, (834), 61–68.
Beketova, M. P., Sokolova, E. A., Malyuchenko, O. P., Alekseev, Y. I., Kuznetsova, M. A., Kozlovsky, B. E., Rogozina, E. V., & Khavkin, E. E. (2014). On molecular identification of Phytophthora infestans genotypes. Russian Agricultural Sciences, 40(6), 435–438.
Brylińska, M., Sobkowiak, S., Stefańczyk, E., & Śliwka, J. (2016). Potato cultivation system affects population structure of Phytophthora infestans. Fungal Ecology, 20, 132–143.
Chowdappa, P., Nirmal Kumar, B. J., Madhura, S., Mohan Kumar, S. P., Myers, K. L., Fry, W. E., & Cooke, D. E. L. (2015). Severe outbreaks of late blight on potato and tomato in South India caused by recent changes in the Phytophthora infestans population. Plant Pathology, 64, 191–199.
Corpet, F. (1988). Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research, 16(22), 10881–10890.
Drenth, A., Janssen, E. M., & Govers, F. (1995). Formation and survival of oospores of Phytophthora infestans under natural conditions. Plant Pathology, 44, 86–94.
Dunn, A. R., Milgroom, M. G., Meitz, J. C., McLeod, A., Fry, W. E., McGrath, M. T., Dillard, H. R., & Smart, C. D. (2010). Population structure and resistance to mefenoxam of Phytophthora capsici in New York state. Plant Disease, 94(12), 1461–1468.
Fernández-Pavía, S. P., Grünwald, N. J., Díaz-Valasis, M., Cadena-Hinojosa, M., & Fry, W. E. (2004). Soilborne oospores of Phytophthora infestans in central Mexico survive winter fallow and infect potato plants in the field. Plant Disease, 88(1), 29–33.
Fry, W. E., Birch, P. R. J., Judelson, H. S., Grünwald, N. J., Danies, G., Everts, K. L., Gevens, A. J., Gugino, B. K., Johnson, D. A., Johnson, S. B., McGrath, M. T., Myers, K. L., Ristaino, J. B., Roberts, P. D., Secor, G., & Smart, C. D. (2015). Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology, 105(7), 966–981.
Fry, W. E., Grünwald, N. J., Cooke, D. E. L., McLeod, A., Forbes, G. A., Cao, K. (2009). Population genetics and population diversity of Phytophthora infestans. In K. Lamour, S. Kamoun (Ed.), Oomycete Genetics and Genomics: Diversity, Interactions, and Research Tools. (pp. 139–164). Wiley-Blackwell.
Fry, W. E., McGrath, M. T., Seaman, A., Zitter, T. A., McLeod, A., Danies, G., Small, I. M., Myers, K., Everts, K., Gevens, A. J., Gugino, B. K., Johnson, S. B., Judelson, H., Ristaino, J., Roberts, P., Secor, G., Seebold, K., Snover-Clift, K., Wyenandt, A., Grünwald, N. J., & Smart, C. D. (2013). The 2009 late blight pandemic in the eastern United States - causes and results. Plant Disease, 97(3), 296–306.
Gallegly, M. E., & Galindo, J. (1958). Mating types and oospores of Phytophthora infestans in nature in Mexico. Phytopathology, 48, 274–277.
Gobena, D., McGrath, M. T., & Lamour, K. (2012). Survival and spread of Phytophthora capsici on Long Island, New York. Mycological Progress, 11, 761–768.
Goss, E. M., Tabima, J. F., Cooke, D. E. L., Restrepo, S., Fry, W. E., Forbes, G. A., Fieland, V. J., Cardenas, M., & Grünwald, N. J. (2014). The Irish potato famine pathogen Phytophthora infestans originated in central Mexico rather than the Andes. Proceedings of the National Academy of Sciences U. S. A, 111(24), 8791–8796.
Govers, F., Drenth, A., & Pieterse, C. M. J. (1997). The potato late blight pathogen Phytophthora infestans and other pathogenic Oomycota. In G. C. Carroll & P. Tudzynski (Eds.), The Mycota V part B (pp. 17–36). Berlin Heidelberg: Springer.
Grünwald, N. J., Garbelotto, M., Goss, E. M., Heungens, K., & Prospero, S. (2012). Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends in Microbiology, 20(3), 131–138.
Harutyunyan, S. R., Zhao, Z., den Hartog, T., Bouwmeester, K., Minnaard, A. J., Feringa, B. L., & Govers, F. (2008). Biologically active Phytophthora mating hormone prepared by catalytic asymmetric total synthesis. Proceedings of the National Academy of Sciences, 105(25), 8507–8512.
Haverkort, A. J., Boonekamp, P. M., Hutten, R., Jacobsen, E., Lotz, L. A. P., Kessel, G. J. T., Visser, R. G. F., & van der Vossen, E. A. G. (2008). Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Research, 51, 47–57.
Hohl, H. R., & Iselin, K. (1984). Strains of Phytophthora infestans with A2 mating type behaviour. Transactions of the British Mycological Society, 83, 529–530.
Hu, J. H., Diao, Y., Zhou, Y., Lin, D., Bi, Y., Pang, Z., Fryxell, R. T., Liu, X., & Lamour, K. (2013). Loss of heterozygosity drives clonal diversity of Phytophthora capsici in China. PLoS One, 8(12), e82691. https://doi.org/10.1371/journal.pone.0082691.
Hurtado-Gonzales, O., Aragon-Caballero, L., Apaza-Tapia, W., Donahoo, R. S., & Lamour, K. H. (2008). Survival and spread of Phytophthora capsici in coastal Peru. Phytopathology, 98, 688–694.
Judelson, H. S. (1996). Chromosomal heteromorphism linked to the mating type locus of the oomycete Phytophthora infestans. Molecular and General Genetics, 252, 155–161.
Judelson, H. S., Spielman, L. J., & Shattock, R. C. (1995). Genetic mapping and non- Mendelian segregation of mating type loci in the oomycete, Phytophthora infestans. Genetics, 141, 503–512.
Jung, T., Colquhoun, I. J., & Hardy, G. E. St. J. (2013). New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. Forest Pathology, 43, 266–288.
Kim, K-J., Eom, S-H., Lee, S-P., Jung, H-S., Kamoun, S., & Lee, Y. S. (2005). A genetic marker associated with the A1 mating type locus in Phytophthora infestans. Journal of Microbiology and Biotechnology, 15(3), 502–509.
Kim, K. J., & Lee, Y. S. (2002). Genetic DNA marker for A2 mating type in Phytophthora infestans. The Journal of Microbiology, 40, 254–259.
Ko, W. H. (1978). Heterothallic Phytophthora: Evidence for hormonal regulation of sexual reproduction. Journal of General Microbiology, 107, 15–18.
Li, Y., Shen, H., Zhou, Q., Qian, K., van der Lee, T., & Huang, S. (2017). Changing ploidy as a strategy: The Irish potato famine pathogen shifts ploidy in relation to its sexuality. Molecular Plant-Microbe Interactions. https://doi.org/10.1094/MPMI-08-16-0156-R.
Mayton, H., Smart, C. D., Moravec, B. C., Mizubuti, E. S. G., Muldoon, A. E., & Fry, W. E. (2000). Oospore survival and pathogenicity of single oospore recombinant progeny from a cross involving US-17 and US-8 genotypes of Phytophthora infestans. Plant Disease, 84(11), 1190–1196.
Mazáková, J., Zouhar, M., Ryšánek, P., Táborský, V., Hausvater, E., & Doležal, P. (2010). Mating type distribution of Phytophthora infestans (Mont.) de Bary in the Czech Republic in 2007 and 2008. Plant Protection Science, 46(3), 89–97.
Meitz, J. C., Linde, C. C., Thompson, A., Langenhoven, S., & McLeod, A. (2010). Phytophthora capsici on vegetable hosts in South Africa: Distribution, host range and genetic diversity. Australasian Plant Pathology, 39, 431–439.
Molli, S. D., Qi, J., Yajima, A., Shikai, K., Imaoka, T., Nukada, T., Yabuta, G., & Ojika, M. (2012). Structure-activity relationship of α hormones, the mating factors of phytopathogen Phytophthora. Bioorganic & Medicinal Chemistry, 20, 681–686.
Niederhauser, J. S. (1956). The blight, the blighter, and the blighted. Transactions of New York. Academy of Sciences, 19, 5–63.
Ojika, M., Molli, S. D., Kanazawa, H., Yajima, A., Toda, K., Nukada, T., Mao, H., Murata, R., Asano, T., Qi, J., & Sakagami, Y. (2011). The second Phytophthora mating hormone defines interspecies biosynthetic crosstalk. Nature Chemical Biology, 7, 591–593.
Qi, J., Asano, T., Jinno, M., Matsui, K., Atsumi, K., Sakagami, Y., & Ojika, M. (2005). Characterization of a Phytophthora mating hormone. Science, 309, 1828.
Shaw, D. S., Fyfe, A. M., Hibberd, P. G., & Abdel-Sattar, M. A. (1985). Occurrence of the rare A2 mating type of Phytophthora infestans on imported Egyptian potatoes and the production of sexual progeny with A1 mating-types from the U.K. Plant Pathology, 34, 552–554.
Śliwka, J., Sobkowiak, S., Lebecka, R., Avendaño-Córcoles, J., & Zimnoch-Guzowska, E. (2006). Mating type, virulence, aggressiveness and metalaxyl resistance of isolates of Phytophthora infestans in Poland. Potato Research, 49, 155–166.
Smart, C. D., & Fry, W. E. (2001). Invasions by the late blight pathogen: Renewed sex and enhanced fitness. Biological Invasions, 3, 235–243.
Smoot, J. J., Gough, F. J., Lamey, H. A., Eichenmuller, J. J., & Gallegly, M. E. (1958). Production and germination of oospores of Phytophthora infestans. Phytopathology, 48, 165–171.
Sujkowski, L. S., Goodwin, S. B., Dyer, A. T., & Fry, W. E. (1994). Increased genotypic diversity via migration and possible occurrence of sexual reproduction of Phytophthora infestans in Poland. Phytopathology, 84, 201–207.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Turkensteen, L. J., Flier, W. G., Wanningen, R., & Mulder, A. (2000). Production, survival and infectivity of oospores of Phytophthora infestans. Plant Pathology, 49, 688–696.
Yajima, A., Qin, Y., Zhou, X., Kawanishi, N., Xiao, X., Wang, J., Zhang, D., Wu, Y., Nukada, T., Yabuta, G., Qi, J., & Asano, T. (2008). Synthesis and absolute configuration of hormone α1. Nature Chemical Biology, 4, 235–237.
Yoshida, K., Schuenemann, V. J., Cano, L. M., Pais, M., Mishra, B., Sharma, R., Lanz, C., Martin, F. N., Kamoun, S., Krause, J., Thines, M., Weigel, D., & Burbano, H. A. (2013). The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. eLife. https://doi.org/10.7554/eLife.00731.
Yuen, J. E., & Andersson, B. (2013). What is the evidence for sexual reproduction of Phytophthora infestans in Europe? Plant Pathology, 62, 485–491.
Zarzycka, H. (2001). Ocena wirulencji, agresywności i typu kojarzeniowego izolatów Phytophthora infestans. IHAR Monografie i Rozprawy Naukowe, 10, 65–67.
Zhang, X. Z., Seo, H. W., Ahn, W. G., & Kim, B. S. (2006). SCAR marker linked with A1 mating type locus in Phytophthora infestans. Journal of Microbiology and Biotechnology, 16(5), 724–730.
Acknowledgements
This work was financed by Polish Ministry of Agriculture and Rural Development, program PW Task 3.1 Monitoring of changes in populations of harmful and quarantine organisms for potato and from a statutory dotation 1-3-00-3-06 from the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The research involved neither human participants nor animals.
Informed consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brylińska, M., Sobkowiak, S., Stefańczyk, E. et al. Evaluation of PCR markers for Phytophthora infestans mating type determination. Eur J Plant Pathol 152, 33–44 (2018). https://doi.org/10.1007/s10658-018-1445-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1445-4