Abstract
Utilizing 32 previously identified S ribonuclease (S-RNase) gene sequences and abundant citrus resources, this study designed specific primers for 10 S-RNase genes. A total of 32 pairs of primers were used to analyze the self-incompatibility genotypes (S-genotypes) of 241 citrus resources, encompassing 105 mandarins, 47 pummelos, 69 oranges, and 20 lemons and citrons. These results provide theoretical guidance for parent selection in production and breeding programs. Among the 215 samples analyzed, two normal S-genotypes were identified, while no S-genotypes were detected in three samples. Notably, 21 samples, primarily citrons, exhibited amplification of only one S-genotype. Additionally, two pummelo samples showed amplification of three S-genotypes each. The integration of S-genotype and selfing phenotype identification revealed five newly discovered self-compatible mutated materials: Changsha ‘Shatian’ pummelo, large-fruited red pummelo, slender leaf ‘Mangshanyegan’, ‘Shatangju’, and W. Murcott. These findings provide valuable resources for investigating the self-compatibility mechanism in citrus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-incompatibility, a genetic mechanism that evolved in flowering plants, aims to prevent inbreeding and maintain species diversity (de Nettancourt 1997; Emerson 1939). Self-incompatibility is classified into sporophytic self-incompatibility (SSI), observed in Brassicaceae plants, and gametophytic self-incompatibility (GSI), found in Solanaceae, Rosaceae, Scrophulariaceae, and others, based on genetic control at the pollen level (Stein et al. 1991; Takayama and Isogai 2005; Wheeler et al. 2009). GSI, primarily mediated by S-RNase-based self-incompatibility, is a prominent mechanism (Huang et al. 1994; Sassa et al. 1997; Xue et al. 1996). This trait is governed by multiple allelic genes at the S locus, where pistil and pollen determinants are closely linked, forming an S haplotype (Aguiar et al. 2013; Foote et al. 1994; Stein et al. 1991). When pollen is deposited onto a pistil with the same S haplotype, self- or non-self-recognition mechanisms inhibit pollen germination or tube elongation, preventing fertilization and resulting in self-incompatibility (Takayama et al. 2000).
Self-incompatibility is pertinent to the citrus industry. Citrus cultivars commonly exhibit self-incompatibility, necessitating planting pollinizer trees or artificial pollination in orchards to achieve consistent fruit set rates and yields (Chai et al. 2010). Therefore, the extensive identification of self-incompatibility genotypes (S-genotypes) in citrus resources holds practical significance in guiding the selection of pollinizer parents.
Early research on self-incompatibility in fruit trees primarily focused on Rosaceae. Significant progress has been made in understanding this mechanism in fruit trees such as pear and apple trees (Long et al. 2010; Verdoodt et al. 1998; Wu et al. 2013). Alongside mechanistic studies, extensive efforts have been dedicated to identifying S-genotypes. While research on self-incompatibility in citrus began later, recent years have seen notable breakthroughs. Liang et al. (2017) conducted groundbreaking genetic, cytological, and in vitro biochemical experiments, demonstrating citrus’s characteristic gametophytic self-incompatibility. They identified S ribonuclease (S-RNase) as the pistil determinant controlling self-incompatibility in citrus, discovering nine alleles of S-RNase. Subsequently, S-genotype analysis was conducted on 394 citrus resources.
Self-incompatibility arises from the interaction between pistil and pollen determinants, and mutation in either can shift it to self-compatibility. In citrus, Liang et al. (2020) identified a naturally occurring S-RNase mutation in self-compatible pummelo. Subsequent testing confirmed self-compatibility in all citrus resources harboring this mutation. Building on this, Hu et al. (2021) investigated the pistil-end mutation underlying self-compatibility in the cultivar ‘Guizhou 1’. Through pollination experiments, transcriptome, and protein expression analyses, they established the absence of S2-RNase expression as the cause of self-compatibility. These findings linked to self-compatibility provide a solid foundation for studying (in) compatibility mechanisms in self-pollinating plants.
This study focused on a diverse collection of citrus resources. Using 32 known sequences of S-RNase genes obtained in our laboratory, specific primers were designed to identify S-genotypes in 241 citrus resources. This comprehensive approach provides a scientific basis for allocating pollination trees in production and selecting parents in breeding programs. Additionally, field experiments involving self-pollination were conducted. Mutated materials exhibiting self-compatibility were identified through the combined analysis of S-genotypes and assessment of self-compatibility phenotypes. These significant findings lay the groundwork for further investigation into the self-incompatibility mechanism in citrus.
Materials and methods
Experimental materials
This study involved 241 citrus resources, comprising 95, 86, and 60 from the Citrus Resource Orchards at Huazhong Agricultural University, the Horticultural Research Institute (Hunan Academy of Agricultural Sciences), and Guangxi Academy of Agricultural Sciences, respectively. The materials encompassed commonly grown commercial varieties and resource varieties, including 105 mandarin, 47 pummelo, 69 orange, and 20 lemon and citron samples. Tender leaves were collected, flash-frozen using liquid nitrogen, and stored at -80 °C.
Self-pollination experiments
From 2020 to 2022, self-pollination experiments were conducted during the peak flowering period at the Citrus Resource Nurseries of the Horticultural Research Institute of Hunan Academy of Agricultural Sciences and Guangxi Institute of Characteristic Crops. After 5 d of pollination, pistils were harvested, fixed, and brought to the laboratory for staining and observation of pollen tube elongation. In total, 66 materials were examined to determine their self-compatible phenotypes, including eight pummelo, 40 mandarin, and 18 orange materials.
Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignments of the S-RNase amino acid sequences were conducted using ClustalX (version 1.83), following the methods outlined by Liang et al. (2017). Amino acid sequence similarity of the S-RNase genes was analyzed using an online tool (https://www.novopro.cn/tools/ident_sim.html). Phylogenetic trees were constructed using Mega X software employing the neighbor-joining method (Kumar et al. 2018). The reliability of the tree was assessed through a 1000 bootstrap replication test.
Design and synthesis of S-genotype specific primers
Thirty-two pairs of specific primers, labeled S1–S31 and Sm, were used to identify S-genotypes. Among them, 10 pairs of primers (Sm and S1–S9) were designed by Dr. Liang Mei from our research team (Liang et al. 2017), 12 (S10–S21) were designed by Zhuangmin Wei from our research team (Wei et al. 2022), and 10 (S22–S31) were newly designed for this study (Table S1). All primers were synthesized by Beijing Tsingke Biotechnology Co., Ltd.
Identification of S-genotypes
Citrus leaf DNA was extracted using the modified CTAB method (Cheng et al. 2005). The quality and concentration of DNA were assessed using a NanoDrop1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). DNA was diluted to a concentration of 200–300 ng·μL−1 and stored at -20 °C. Gene amplification followed methods from previous studies conducted in our laboratory (Chai et al. 2011). The PCR mix (2 × Hieff® PCR Master Mix [With Dye], Cat No. 10102ES03) was purchased from Yeasen Biotechnology Co. (Shanghai, China). S-genotypes of the identified materials were determined by analyzing the size of PCR-amplified fragments, with results subjected to statistical analysis.
Identification of self-compatibility phenotypes
Self-pollination
During the peak flowering period of citrus on sunny, windless days, 15 unopened buds at the late balloon stage were selected. Petals were carefully removed using forceps, and fresh pollen was collected with a pollination brush and deposited onto the stigma. Flowers were subsequently bagged and labeled with the variety name, number of pollinations, and pollination time.
Fixation and preservation
Six days post-self-pollination, pollinated stigmas were collected and immersed in a fixative solution for 12 h. After fixation, they were washed 2–3 times with a 95% ethanol solution and stored in a 70% ethanol solution.
Staining and observation
Preserved stigma samples were washed with distilled water, immersed in a 4 mol/L NaOH solution, and placed in a water bath at 65℃. Duration varied depending on stigma size, with grapefruit stigmas requiring 60 min and others requiring 10–20 min. After softening, stigmas were rinsed 3–4 times with distilled water, soaked in an aniline blue solution for 12 h for staining, and longitudinally divided into two equal halves using a surgical blade. One half was mounted on a glass slide with 2–3 drops of polyethylene glycol and observed under a microscope using a 5 × objective lens. For each variety, five individual stigmas were observed. Affinity was inferred if pollen tubes grew below the stigma’s surface; nonaffinity was concluded if pollen tubes failed to reach the stigma’s base. Confocal laser scanning microscopy (Leica TCS SP8, Leica Microsystemes, Wetzlar, Germany) was employed to observe and capture images of pollen tube growth in both stigma and style, which were documented as part of the experiment. These methods were referenced from previous studies (Liang et al. 2017).
Results
Diversity and phylogenetic analysis of S-RNase sequences
All S-RNase genes analyzed in this study have been previously reported. S1-RNase to S14-RNase and Sm-RNase were initially reported by Liang et al. (2020) with National Center for Biotechnology Information (NCBI) accession numbers of MN652897 to MN652911; S15-RNase to S17-RNase were first reported by Honsho et al. (2021), with NCBI accession numbers LC575202, LC575207, and LC575209, respectively; and S18-RNase to S31-RNase were first reported by Hu et al. (2023), with NCBI accession numbers ON227020 to ON227033. Detailed gene information is provided in Table S2.
Based on previous research by our group (Liang et al. 2020; Wei et al. 2022; Hu et al. 2023), we performed sequence alignment and phylogenetic analysis of 10 genes (S22–S31) that had not yet been analyzed. Sequence information for these 10 genes can be found in Dataset S1. Our analysis revealed a high level of polymorphisms among the 10 S-RNase alleles within the population, with amino acid similarity ranging from 45.20% to 93.81% (Table S3). Upon analyzing their sequences, we found that the size of these 10 genes was similar, each containing five conserved regions (C1–C5) and two hypervariable regions (Hva and Hvb) (Fig. 1). We constructed a phylogenetic tree of citrus S-RNase alleles through multiple sequence alignment analysis. The results showed that the 32 S-RNase alleles displayed either close or distant evolutionary relationships. However, no significant differences were observed among different citrus species. The clustering of S-RNase alleles identified in different citrus species showed no clear boundaries and overlapped with each other (Fig. 2).
Analysis of S-genotypes in 105 mandarin samples
S-genotype identification among 105 mandarin samples revealed complete amplification of both S-genotypes in 93 samples. Nine samples exhibited amplification of only one S-genotype, whereas three samples did not show any amplification of S-genotypes (Figure S1). The results are presented in Table 1. All 23 samples, including ‘Huanong Bendizao’, were identified as having the S9Sm genotype, while another set of 23 samples, including ‘Huanong’ seedless Ponkan, exhibited the S2Sm genotype. Of the 32 S-RNase sequences, 17 were successfully amplified in mandarins. Sm-RNase was the most frequently detected allele, amplified in 63 samples at a frequency of 60%. Following this, S9-RNase, S2-RNase, and S7-RNase were detected in 29, 22, and 16 samples, respectively, with frequencies of 28%, 21%, and 15%. Frequencies of S11-RNase, S16-RNase, S30-RNase, and S31-RNase ranged between 9.5% and 12.3%. S10-RNase, S21-RNase, S25-RNase, and S26-RNase were detected at frequencies ranging from 1.9% to 4.8%. The remaining alleles (S3-RNase, S5-RNase, S15-RNase, S23-RNase, and S27-RNase) were detected at a frequency of 0.95% (Fig. 4).
S-genotype analysis of 47 pummelo samples
In the analysis of S-genotypes among 47 pummelo samples, 42 samples exhibited amplification of two S-RNase alleles (Fig. 3). Additionally, two samples displayed amplification of three S-RNase alleles: ‘Chenzhou’ pummelo (S3S7S19) and ‘Jiangyong’ pummelo (S1S2S19). Moreover, three samples exhibited amplification of only a single S-RNase allele: ‘Huanong’ red pummelo (S9), ‘Jiangyong’ sour pummelo (S6), and ‘Xiachang’ pummelo (S4) (Table 2). Among the 32 S-RNase alleles, 20 were successfully amplified in the pummelo resources, representing the category with the highest number of different S-RNase alleles among the identified citrus resources. Notably, 14 materials exhibited amplification of the S2-RNase allele, showing the highest frequency at 29.8%. Additionally, nine materials showed amplification of the S3-RNase allele, whereas seven materials displayed amplification of the S5-RNase allele, with frequencies of 19.1% and 14.9%, respectively. The amplification frequencies for S1-RNase, S4-RNase, S5-RNase, S9-RNase, S10-RNase, S16-RNase, S19-RNase, S20-RNase, S21-RNase, S22-RNase, and S24-RNase ranged from 8.5% to 12.7%. Conversely, the amplification frequencies for S6-RNase, S7-RNase, S8-RNase, S18-RNase, S23-RNase, S25-RNase, and S27-RNase ranged from 2.1% to 6.4% (Fig. 4).
Distribution of S-RNase gene frequencies across 241 citrus materials. The bar graph illustrates the frequency distribution of each S-genotype among various types of citrus. The y-axis represents the frequency, while the x-axis represents the different S-genotypes. Mandarins are depicted in purple, pummelos in orange, oranges in green, and lemons and citrons in yellow. S-RNase, Stylar Ribonuclease; S1–S31, S1-RNase–S31-RNase
S-genotype analysis of 69 orange samples
Among the 69 orange samples collected, samples numbered 1–14 are classified as sour oranges, while samples numbered 15–69 are categorized as sweet oranges. Upon identifying the S-genotypes of these oranges, 68 materials, except for ‘seedless sour orange’, exhibited amplification of two S-genotypes. Excluding ‘Bingtang’ orange and ‘Jinshan’ orange, Sm-RNase was amplified in 67 of the materials. Among the 55 sweet oranges, 46 materials, including ‘Hamlin’ sweet orange, were identified as having the S7Sm genotype (Figure S2). Detailed results are presented in Table 3.
Out of the 32 S-RNase alleles, 12 were successfully amplified in oranges. Sm-RNase had the highest frequency at 97% among these alleles. S7-RNase was the next most frequent allele, with a frequency of 75%. S9-RNase and S26-RNase both had a frequency of 4.35%. S2-RNase, S19-RNase, and S21-RNase each had a frequency of 2.90%. S3-RNase, S4-RNase, S16-RNase, S22-RNase, and S25-RNase were amplified in only one sample each, with a frequency of 1.45% (Fig. 4).
S-genotype exploration in lemon and citron samples
After identifying S-genotypes in 20 lemon and citron samples (Figure S3), 10 lemon samples exhibited amplification of two S-RNase alleles each. Of the 10 citron samples, only two possessed two S-RNase alleles: ‘Jinhua’ citron (S8S21) and ‘Xizang’ citron 1 (S14S21). The remaining eight citron samples exhibited amplification of only one S-RNase allele: S8-RNase (Table 4). Of the 20 samples, nine S-RNase alleles were amplified. Notably, the S8-RNase allele was detected in 17 samples, accounting for a frequency of 85%. The frequencies of the remaining S-RNase alleles ranged from 5 to 15% (Fig. 4).
Self-compatibility phenotype of 66 citrus materials
In total, 66 citrus materials were selected randomly for self-pollination, with the self-compatibility phenotypes detailed in Table 5. Through self-pollination, ‘Changsha Shatian’ pummelo and large-fruited red pummelo were identified as self-compatible among the eight pummelo materials tested. Among the 40 mandarin materials, 16 were identified as self-compatible, including Sm types, while all Valencia oranges demonstrated self-compatibility. Both Satsuma orange and navel orange displayed male sterility, resulting in limited pollen tube elongation. After comprehensive S-genotype identification and self-compatibility phenotype assessment over two years or more, five new self-compatible mutant materials (non-Sm mutations) were discovered. These materials include ‘Changsha Shatian’ pummelo, large-fruited red pummelo, slender leaf ‘Mangshanyegan’, ‘Shatangju’, and W. Murcott. Aniline blue staining of the styles can be found in Fig. 5.
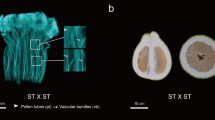
Aniline blue staining results for five self-compatible citrus materials. Aniline blue staining was performed on the stigmas of five recently discovered self-compatible citrus cultivars. The results are presented in panels a, b, c, d, and e, corresponding to ‘Changsha Shatian’ Pummelo, large-fruited red pummelo, slender leaf ‘Mangshanyegan’, ‘Shatangju’, W. Murcott, respectively. Panel f represents the control of self-incompatibility. The red arrow indicates the pollen tubes (pt), and the yellow arrow indicates the vascular bundles (vb). Yellow lines represent bar = 1 μm. The results depicted in the figures demonstrate self-compatibility across all the cultivars
Discussion
In this study, 32 pairs of S-genotype specific primers were used, with 10 pairs (S22–S31) reported for the first time. These primers were designed based on the newly discovered S-RNase gene sequences discovered. We identified S-genotypes from 241 different citrus resources, including mandarins, pummelos, oranges, lemons, and citrons. The results indicated that the 32 S-RNase genes covered nearly all the citrus resources examined in this research. However, a few materials remained unidentified for their S-genotypes, suggesting the potential existence of undiscovered S-RNase genes in citrus. Furthermore, some materials exhibited only one identified S-genotype, suggesting they may either be homozygotes with a single S-RNase gene or possess an additional undiscovered S-RNase gene.
The S-genotype identification revealed the amplification of 20 distinct S-RNase genes in pummelos and 17 in mandarins, indicating the highest richness of S-genotypes in these categories. This richness could be attributed to their high heterozygosity within populations and the abundant genetic diversity among varieties. Interestingly, none of the pummelos and citrons harbored the Sm-RNase gene, whereas mandarins exhibited the highest amplification frequency for this gene. Among pummelos, the S2-RNase gene displayed the highest amplification frequency, consistent with previous findings (Liang et al. 2020). Notably, two pummelos exhibited amplification of three different S-RNase genes, warranting further investigation into this finding.
Of the 69 orange samples, 67 exhibited amplifications of the Sm-RNase gene, with 46 sweet orange materials having an identified S7Sm genotype. Sweet oranges, hybrid descendants of pummelos and mandarins, may have inherited shoot mutations, possibly explaining the prevalence of the S7Sm genotype in this category.
Lemons and citrons showed the highest amplification frequency of the S8-RNase gene. Notably, only the S8-RNase S-genotype was identified in some citrons produced in Yunnan and Xizang. Citrons, an earlier-diverging citrus species with a relatively stable genetic background, might exhibit homozygosity of their S-genotypes.
In this study, self-compatibility phenotype was identified among 66 samples. Combining the S-genotype identification results, two new affinity mutant materials ('Changsha Shatian' pummelo and large-fruited red pummelo) were discovered among pummelos. Among mandarins, 16 self-compatible materials were identified, with six materials ('Gonggan' and others) classified as Sm-type affinity mutants. However, the S-genotypes of 'Chunjian', 'Ganping', and 'Lihuaju' remain unknown and necessitate further identification to determine whether they represent new affinity materials. After two years of consecutive self-pollination, two new affinity materials—'Shatangju' and W. Murcott, were discovered in mandarins.
The study highlights the presence of two distinct cultivars of 'Mangshanyegan', a relatively ancient citrus variety (round leaf and slender leaf) with differing S-genotypes and levels of self-compatibility. Further studies are required to validate the phenotype identification results and conduct in-depth investigations. Despite advancements, the mechanism underlying self-incompatibility in citrus remains poorly understood. The discovery of affinity mutant materials holds promise for uncovering new insights into citrus self-incompatibility mechanisms, thereby facilitating further research in this area.
Conclusions
To sum up, this study employed specific primers to identify S-genotypes in various citrus fruits. The findings unveiled a wide range of S-genotypes across mandarins, pummelos, oranges, lemons, and citrons, with pummelos and mandarins exhibiting the highest diversity. However, some samples remained unidentified for their S-genotypes, suggesting the potential existence of undiscovered S-RNase genes in citrus. Additionally, certain samples displayed only one identified S-genotype, indicating possible homozygosity or the presence of undiscovered S-RNase genes. Therefore, S-genotypes in citrus can be further mined by transcriptome sequencing in subsequent studies. Notably, the study discovered new affinity mutant materials among pummelos and mandarins, underscoring the need for further investigation. Furthermore, distinct cultivars of 'Mangshanyegan' with varying S-genotypes and levels of self-compatibility were revealed. While these findings contribute to our understanding of citrus self-incompatibility mechanisms, further research is imperative to validate and expand upon these results.
Availability of data and materials
Data will be available on request.
References
Aguiar B, Vieira J, Cunha AE, Fonseca NA, Reboiro-Jato D, Reboiro-Jato M, et al. Patterns of evolution at the gametophytic self-incompatibility Sorbus aucuparia (Pyrinae) S pollen genes support the non-self recognition by multiple factors model. J Exp Bot. 2013;64:2423–34. https://doi.org/10.1093/jxb/ert098.
Chai L, Biswas MK, Ge X, Deng X. Isolation, characterization, and expression analysis of an SKP1-like gene from ‘Shatian’ Pummelo (Citrus grandis Osbeck). Plant Mol Biol Rep. 2010;28:569–77. https://doi.org/10.1007/s11105-010-0184-2.
Chai L, Ge X, Xu Q, Deng X. CgSL2, an S-like RNase gene in ‘Zigui shatian’ pummelo (Citrus grandis Osbeck), is involved in ovary senescence. Mol Biol Rep. 2011;38:1–8. https://doi.org/10.1007/s11033-010-0070-x.
Cheng Y, De Vicente MC, Meng H, Guo W, Tao N, Deng X. A set of primers for analyzing chloroplast DNA diversity in citrus and related genera. Tree Physiol. 2005;25:661–72. https://doi.org/10.1093/treephys/25.6.661.
De Nettancourt D. Incompatibility in angiosperms. Sex Plant Reprod. 1997;10:185–99. https://doi.org/10.1007/s004970050087.
Emerson S. A preliminary survey of the oenothera organensis population. Genetics. 1939;24:524–37. https://doi.org/10.1093/genetics/24.4.524.
Foote HCC, Ride JP, Franklin-Tong VE, Walker EA, Lawrence MJ, Franklin FCH. Cloning and expression of a distinctive class of self-incompatibility (S) gene from Papaver rhoeas L. Proc Natl Acad Sci USA. 1994;91:2265–9. https://doi.org/10.1073/pnas.91.6.2265.
Honsho C, Ushijima K, Anraku M, Ishimura S, Yu Q, Gmitter FG, et al. Association of T2/S-RNase with self-incompatibility of Japanese citrus accessions examined by transcriptomic, phylogenetic, and genetic approaches. Front Plant Sci. 2021;12:638321. https://doi.org/10.3389/fpls.2021.638321.
Hu J, Xu Q, Liu C, Liu B, Deng C, Chen C, et al. Downregulated expression of S(2)-RNase attenuates self-incompatibility in “Guiyou No. 1” pummelo. Hortic Res. 2021;8:199. https://doi.org/10.1038/s41438-021-00634-8.
Hu J, Liu C, Du Z, Guo F, Song D, Wang N, et al. Transposable elements cause the loss of self-incompatibility in citrus. Plant Biotechnol J. 2023;12:1113–31. https://doi.org/10.1111/pbi.14250.
Huang S, Lee HS, Karunanandaa B, Kao TH. Ribonuclease activity of Petunia inflata S proteins is essential for rejection of self-pollen. Plant Cell. 1994;6:1021–8. https://doi.org/10.1105/tpc.6.7.1021.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. https://doi.org/10.1093/molbev/msy096.
Liang M, Yang W, Su S, Fu L, Yi H, Chen C, et al. Genome-wide identification and functional analysis of S-RNase involved in the self-incompatibility of citrus. Mol Genet Genomics. 2017;292:325–41. https://doi.org/10.1007/s00438-016-1279-8.
Liang M, Cao Z, Zhu A, Liu Y, Tao M, Yang H, et al. Evolution of self-compatibility by a mutant S(m)-RNase in citrus. Nat Plants. 2020;6:131–42. https://doi.org/10.1038/s41477-020-0597-3.
Long S, Li M, Han Z, Wang K, Li T. Characterization of three new S-alleles and development of an S-allele-specific PCR system for rapidly identifying the S-genotype in apple cultivars. Tree Genet Genomes. 2010;6:161–8. https://doi.org/10.1007/s11295-009-0237-6.
Sassa H, Hirano H, Nishio T, Koba T. Style-specific self-compatible mutation caused by deletion of the S-RNase gene in Japanese pear (Pyrus serotina). Plant J. 1997;12:223–7. https://doi.org/10.1046/j.1365-313X.1997.12010223.x.
Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA. 1991;88:8816–20. https://doi.org/10.1073/pnas.88.19.8816.
Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467–89. https://doi.org/10.1146/annurev.arplant.56.032604.144249.
Takayama S, Shiba H, Iwano M, Shimosato H, Che FS, Kai N, et al. The pollen determinant of self-incompatibility in brassica campestris. Proc Natl Acad Sci USA. 2000;97:1920–5. https://doi.org/10.1073/pnas.040556397.
Verdoodt L, Van Haute A, Goderis IJ, De Witte K, Keulemans J, Broothaerts W. Use of the multi-allelic self-incompatibility gene in apple to assess homozygocity in shoots obtained through haploid induction. Theor Appl Genet. 1998;96:294–300. https://doi.org/10.1007/s001220050739.
Wei Z, Wei S, Chen P, Hu J, Tang Y, Ye J, et al. Identification of self-incompatibility genotypes (S-genotypes) of 63 pummelo germplasm resources. Acta Hortic Sin. 2022;49:1111–20. https://www.ahs.ac.cn/CN/10.16420/j.issn.0513-353x.2021-0260.
Wheeler MJ, de Graaf BHJ, Hadjiosif N, Perry RM, Poulter NS, Osman K, et al. Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature. 2009;459:992–5. https://doi.org/10.1038/nature08027.
Wu J, Li M, Li T. Genetic features of the spontaneous self-compatible mutant, ‘Jin Zhui’ (Pyrus bretschneideri Rehd.). PLoS One. 2013;8:e76509. https://doi.org/10.1371/journal.pone.0076509.
Xue Y, Carpenter R, Dickinson HG, Coen ES. Origin of allelic diversity in antirrhinum S locus RNases. Plant Cell. 1996;8:805–14. https://doi.org/10.1105/tpc.8.5.805.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Research and Development Program of Guangdong Province (2022B0202070002), the National Natural Science Foundation of China (32122075, 32072523), the National Modern Agricultural (Citrus) Industry Technology System (CARS-27), the Special Project for the Construction of Innovative Provinces in Hunan Province (2022SK2140), and the Natural Science Foundation of Changsha (kq2208136).
Author information
Authors and Affiliations
Contributions
S.D., P.K., H.J., C.P. and C.C. conducted the experiments. Y.J., X.Z. and D.X. provided guidance for this study. C.G. and S.D. performed data analysis and manuscript writing. C.G. and C.L. edited the manuscript. All authors read and approved the final manuscript. C.G., S.D., and P.K. contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest. Prof. Xiuxin Deng is the Editor-in-Chief of Horticulture Advances and was not involved in the journal’s review or decisions related to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44281_2024_35_MOESM1_ESM.tif
Supplementary Material 1: Figure S1. PCR results of 105 mandarins. The agarose 1.2% gel depicts the amplified S-genotype profiles of 105 mandarins. Each number from 1 to 105 corresponds to a specific mandarin species. S-RNase, Stylar Ribonuclease; S1–S31, S1-RNase–S31-RNase.
44281_2024_35_MOESM2_ESM.tif
Supplementary Material 2: Figure S2. PCR results of 69 types of oranges. The agarose 1.2% gel depicts the amplified S-genotype profiles of 69 types of oranges. Each number from 1 to 69 corresponds to a specific orange type. S-RNase, Stylar Ribonuclease; S1–S31, S1-RNase–S31-RNase.
44281_2024_35_MOESM3_ESM.tif
Supplementary Material 3: Figure S3. PCR results of 20 lemons and citrons. The agarose 1.2% gel depicts the amplified S-genotype profiles of 20 lemons and citrons. Each number from 1 to 20 corresponds to a specific lemon or citron species. S-RNase, Stylar Ribonuclease; S1–S31, S1-RNase–S31-RNase.
44281_2024_35_MOESM7_ESM.docx
Supplementary Material 7: Dataset S1. Sequence information for S22-RNase, S23-RNase, S24-RNase, S25-RNase, S26-RNase, S27-RNase, S28-RNase, S29-RNase, S30-RNase, and S31-Rnase.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cai, G., Song, D., Peng, K. et al. Identifying citrus self-incompatibility genotypes (S-genotypes) and discovering self-compatible mutants. HORTIC. ADV. 2, 20 (2024). https://doi.org/10.1007/s44281-024-00035-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44281-024-00035-6