Abstract
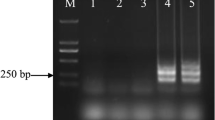
Anguina wevelli is a pathogenic grass parasitic nematode, however it is difficult to identify based simply on morphology. This study developed a loop-mediated isothermal amplification (LAMP) assay to detect A. wevelli. The LAMP method developed could specifically detect A. wevelli in 45 min, and the detection limit was 1/80000 of the total DNA extracted from a single juvenile (J2), an equivalent of 2.5 pg of DNA. This is the first report of the detection of Anguina spp. by using a LAMP-based method.



Similar content being viewed by others
References
Chizhov, V. N., & Subbotin, S. A. (1990). Plant-parasitic nematodes of the subfamily Anguininae (Nematoda, Tylenchida). Morphology, trophic specialization, systematics. Zoologicheskiĭ Zhurnal, 69(1), 15–26.
Duan, Y. B., Ge, C. Y., Zhang, X. K., Wang, J. X., & Zhou, M. G. (2014). Development and evaluation of a novel and rapid detection assay for Botrytis Cinerea based on loop-mediated isothermal amplification. PloS One, 9(10), e111094.
Edgar, J. A., Frahn, J. L., Cockrum, P. A., Anderton, N., Jago, M. V., Culvenor, C. C., Jones, A. J., Murray, K., & Shaw, K. J. (1982). Corynetoxins, causative agents of annual ryegrass toxicity; their identification as tunicamycin group antibiotics. Journal of the Chemical Society Chemical Communication, 4(4), 222–224.
Gasser, R. B., & Newton, S. E. (2000). Genomic and genetic research on bursate nematodes: significance, implications and prospects. International Journal for Parasitology, 30(4), 509–534.
Griesbach, J., Chitambar, J. J., Hamerlynck, M. J., & Duarte, E. O. (1999). A comparative analysis of extraction methods for the recovery of Anguina sp. from grass seed samples. Journal of Nematology, 33, 635–640.
Iwamoto, T., Sonobe, T., & Hayashi, K. (2003). Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare. Journal of Clinical Microbiology, 41(6), 2616–2622.
Kikuchi, T., Aikawa, T., Oeda, Y., Karim, N., & Kanzaki, N. (2009). A rapid and precise diagnostic method for detecting the pinewood nematode Bursaphelenchus xylophilus by loop-mediated isothermal amplification. Phytopathology, 99(12), 1365–1369.
Krall, E. L. (1991). Wheat and grass nematodes: Anguina, Subanguina, and related genera. In W. R. Nickle (Ed.), Manual of agricultural nematology (pp. 721–760). New York: Marcel Dekkersss.
Li, W. B., Yan, Z. H., & Nakhla, M. K. (2015). Real-time PCR for detection and identification of Anguina funesta, A. agrostis, A. tritici, and A. pacificae. Plant Disease, 99(11), 1584–1589.
Lin, B. R., Wang, H. H., Zhuo, K., & Liao, J. L. (2016). Loop-mediated isothermal amplification for the detection of Tylenchulus semipenetrans in soil. Plant Disease, 100(5), 877–883.
Liu, X. T., Wang, H. H., Lin, B. R., Tao, Y., Zhuo, K., & Liao, J. L. (2016). Loop-mediated isothermal amplification based on the mitochondrial COI region to detect Pratylenchus zeae. European Journal of Plant Pathology. doi:10.1007/s10658-016-1102-8.
Ma, Y. G., Wang, J. C., Xie, H., Zhou, C. N., Du, Y., Huang, G. M., & Li, F. R. (2006). Multiple-PCR detection on three species of Anguina genus. Acta Phytopathologica Sinica, 6, 508–511.
Maeda, H., Kokeguchi, S., Fujimoto, C., Tanimoto, I., Yoshizumi, W., Nishimura, F., & Takashiba, S. (2005). Detection of periodontal pathogen Porphyromonas gingivalis by loopmediated isothermal amplification method. FEMS Immunology and Medical Microbiology, 43(2), 233–239.
Mori, Y., Nagamine, K., Tomita, N., & Notomi, T. (2001). Detection of loop mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochemical and Biophysical Research Communications, 289, 150–154.
Nagamine, K., Hase, T., & Notomi, T. (2002). Accelerated reaction by loop mediated isothermal amplification using loop primers. Molecular and Cellular Probes, 16, 223–229.
Niu, J. H., Jian, H., Guo, Q. X., Chen, C. L., Wang, X. Y., Liu, Q., & Guo, Y. D. (2012). Evaluation of loop-mediated isothermal amplification (LAMP) assays based on 5S rDNA-IGS2 regions for detecting Meloidogyne enterolobii. Plant Pathology, 61(4), 809–819.
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000). Loop mediated isothermal amplification of DNA. Nucleic Acids Research, 28(12), e63.
Peng, J., Shi, M., Xia, Z., Huang, J., & Fan, Z. (2012). Detection of cucumber mosaic virus isolates from banana by one-step reverse transcription loop-mediated isothermal amplification. Archives of Virology, 157, 2213–2217.
Powers, T. O., Todd, T. C., Burnell, A. M., Murray, P. C. B., Fleming, C. C., Szalanski, A. L., Adams, B. A., & Harris, T. S. (1997). The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. Journal of Nematology, 29(4), 441–450.
Powers, T. O., Szalanski, A. L., Mullin, P. G., Harris, T. S., Bertozzi, T., & Griesbach, J. A. (2001). Identification of seed gall nematodes of agronomic and regulatory concern with PCR-RFLP of ITS1. Journal of Nematology, 33(4), 191–194.
Price, P. C., Fisher, J. M., & Kerr, A. (1979). One Anguina funesta n. sp. and its association with Corynebacterium sp. in infecting Lolium rigidum. Nematologica, 25, 76–85.
Riley, I. T. (1992). Anguina tritici is a potential vector of Clavibacter toxicus. Australasian Plant Pathology, 21(4), 147–149.
Riley, I. T., Schmitz, A., & Silva, P. (2001). Anguina australis, a vector for Rathayibacter toxicus in Ehrharta longiflora. Australasian Plant Pathology, 30, 171–175.
Shina, S., Revathy, K. A., & Bhat, A. I. (2015). Rapid identification of transgenic black pepper using loop-mediated isothermal amplification (LAMP) and real-time LAMP assays. Journal of Plant Biochemistry Biotechnology, 24(4), 466–469.
Siddiqi, M. R. (2000). Tylenchida: Parasites of plants and insects (2nd ed.). Oxon: CABI Publishing, CAB International.
Song, Q. Q., Wang, L. X., Fang, R., Khan, M. K., Zhou, Y. Q., & Zhao, J. L. (2013). Detection of Mycoplasma wenyonii in cattle and transmission vectors by the loop-mediated isothermal amplification (LAMP) assay. Tropical Animal Health and Production, 45, 247–250.
Stynes, B. A., & Bird, A. F. (1980). Anguina agrostis, the vector of annual ryegrass toxicity in Australia. Nematologica, 26, 475–490.
Subbotin, S. A., Waeyenberge, L., & Moens, M. (2000). Identification of cyst forming nematodes of the genus Heterodera (Nematoda: Heteroderidae) based on the ribosomal DNA-RFLPs. Nematology, 2(2), 153–164.
Subbotin, S. A., Krall, E. L., Riley, I. T., Chizhov, V. N., Staelens, A., De Loose, M., & Moens, M. (2004). Evolution of the gall-forming plant parasitic nematodes (Tylenchida:Anguinidae) and their relationships with hosts as inferred from internal transcribed spacer sequences of nuclear ribosomal DNA. Molecular Phylogenetics and Evolution, 30(1), 226–235.
Tomlinson, J. A., Boonham, N., & Dickinson, M. (2010). Development of and evaluation of a one hour DNA extraction and loop mediated isothermal amplification assay for rapid detection of phytoplasmas. Plant Pathology, 56, 465–471.
Wang, J. C., Ma, Y. G., Zhou, C. N., Du, Y., Huang, G. M., Ding, Y. M., & Xie, H. (2005). Molecular identification bent grass nematode, by the single juvenile. Plant Quarantine, 19(2), 84–86 (in Chinese).
Xie, H. (2005). Taxonomy of plant nematodes (Second ed.). Beijing: Higher Education Press.
Zhang, J. H., Feng, Y. J., Hu, D., Lv, H., Zhu, J., Cao, M., Zheng, F., Zhu, J., Gong, X. F., Hao, L. N., Srinivas, S., Ren, H., Qi, Z. T., Li, B. J., & Wang, C. J. (2013). Rapid and sensitive detection of H7N9 avian influenza virus by use of reverse transcription–loop-mediated isothermal amplification. Journal of Clinical Microbiology, 51(11), 3760–3764.
Zhou, Q. J., Cai, Y., Gu, J. F., Wang, X., & Chen, J. (2016). Rapid and sensitive detection of Meloidogyne mali by loop-mediated isothermal amplification combined with a lateral flow dipstick. European Journal of Plant Pathology. doi:10.1007/s10658-016-1130-4.
Acknowledgements
The project was supported by the Scientific Research Project of Shanghai Entry -Exit Inspection and Quarantine Bureau (No. HK004-2014 and No. HK003-2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The manuscript has not been submitted to more than one journal for simultaneous consideration.
The manuscript has not been published previously (partly or in full).
A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (e.g. “salami-publishing”).
No data have been fabricated or manipulated (including images) to support your conclusions.
No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”). Proper acknowledgements to other works must be given (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), quotation marks are used for verbatim copying of material, and permissions are secured for material that is copyrighted.
Important note: the journal may use software to screen for plagiarism.
Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities - tacitly or explicitly - at the institute/organization where the work has been carried out, before the work is submitted.
Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Authors are strongly advised to ensure the correct author group, corresponding author, and order of authors at submission. Changes of authorship or in the order of authors are not accepted after acceptance of a manuscript.
Rights and permissions
About this article
Cite this article
Yu, Lz., Song, Sy., Yu, C. et al. A loop mediated isothermal amplification (LAMP) assay for rapid and reliable detection of Anguina wevelli, a grass parasitic nematode. Eur J Plant Pathol 150, 725–734 (2018). https://doi.org/10.1007/s10658-017-1320-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1320-8