Abstract
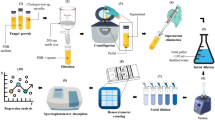
Long-lasting viable fungal spores are one of the important aspects in emergence, spread and disease development of pathogenic fungi. We developed a rapid and miniaturized system using Alamar Blue (resazurin dye; 7-hydroxy-3H-phenoxazin-3-one 10-oxide) for assessing fungal spore viability, using the ascomycete Leptosphaeria maculans (causing blackleg disease on canola) as a ‘model pathogen’. The assay is dependent on the metabolic activity of viable fungal spores to convert the dark blue of resazurin (maximum absorbance 605 nm) into the pink colour of resorufin (maximum absorbance 573 nm). The Alamar Blue assay uses an optimised micro-titre based format that was far superior for determining fungal spore viability in comparison with current conventional techniques including trypan blue staining, a TC10 cellometer cell counter, or by assessing germination of the spores under the microscope. This new assay was also more rapid and reproducible than current conventional tests to detect viable spores. Viable spores could be reliably detected within two hours. The successful application of the Alamar Blue assay to measure fungal spore viability in the current study has important benefits for biosecurity operations relating to faster and more reliable confirmation of viability of potential invasive exotic fungal pathogens and in minimising any consequent disease outbreaks. The effectiveness of the Alamar Blue assay was confirmed by successfully determining the relative retention times of viable L. maculans ascospores across a range of different potential spore-carrier materials, including steel, fabric, wood, paper, rubber and leather, over a time period of eight months. To further confirm the wide applicability of the Alamar Blue assay, it was successfully applied to detect viable spores of fungal pathogens of diverse taxonomic groups, including Kabatiella caulivora, Magnaporthe oryzae and Puccinia striiformis f.sp. tritici, and also of the yeast Saccharomyces cerevisiae.




Similar content being viewed by others
References
Ahmed, S. A., Gogal, R. M., & Walsh, J. E. (1994). A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H] thymidine incorporation assay. Journal of Immunological Methods, 170, 211–224.
Al-Nasiry, S., Geusens, N., Hanssens, M., Luyten, C., & Pijnenborg, R. (2007). The use of Alamar blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Human Reproduction, 22, 1304–1309.
Barbetti, M. J. (1995). Breakdown in resistance of subterranean clovers to clover scorch disease (Kabatiella caulivora). Australian Journal of Agricultural Research, 46, 645–653.
Barbetti, M. J., & Khangura, R. K. (1999). Managing blackleg in the disease-prone environment of Western Australia. pp. 100,In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia.
Bebber, D. P., & Gurr, S. J. (2015). Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genetics and Biology, 74, 62–64.
Bio-Rad (2010). TC10™Automated cell counter instruction manual. (pp. 11–16).
Borra, R. C., Lotufo, M. A., Gagioti, S. M., Barros, F. d. M., & Andrade, P. M. (2009). A simple method to measure cell viability in proliferation and cytotoxicity assays. Brazilian Oral Research, 23, 255–262.
Bowling, T., Mercer, L., Don, R., Jacobs, R., & Nare, B. (2012). Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. International Journal for Parasitology: Drugs and Drug Resistance, 2, 262–270.
Brown, J. K., & Hovmøller, M. S. (2002). Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science, 297, 537–541.
Bueno, C., Villegas, M. L., Bertolotti, S. G., Previtali, C. M., Neumann, M. G., & Encinas, M. V. (2002). The excited-state interaction of resazurin and resorufin with aminesin aqueous solutions. Photophysics and photochemical reaction. Photochemistry and Photobiology, 76, 385–390.
Carnegie, A. J., & Cooper, K. (2011). Emergency response to the incursion of an exotic myrtaceous rust in Australia. Australasian Plant Pathology, 40, 346–359.
Castilho, A. L., Caleffi-Ferracioli, K. R., Canezin, P. H., Dias Siqueira, V. L., de Lima Scodro, R. B., & Cardoso, R. F. (2015). Detection of drug susceptibility in rapidly growing mycobacteria by resazurin broth microdilution assay. Journal of Microbiological Methods, 111, 119–121.
Chadha, S., & Kale, S. P. (2015). Simple fluorescence-based high throughput cell viability assay for filamentous fungi. Letters in Applied Microbiology, 61, 238–244.
Coban, A. Y., Deveci, A., Sunter, A. T., Palomino, J. C., & Martin, A. (2014). Resazurin microtiter assay for isoniazid, rifampicin, ethambutol and streptomycin resistance detection in Mycobacterium tuberculosis: updated meta-analysis. International Journal of Mycobacteriology, 3, 230–241.
Crone, M., McComb, J. A., O’Brien, P. A., & Hardy, G. E. S. J. (2013). Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology, 117, 112–123.
da Cruz Cabral, L., Fernández Pinto, V., & Patriarca, A. (2013). Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. International Journal of Food Microbiology, 166, 1–14.
Fai, P. B., & Grant, A. (2009). A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere, 74, 1165–1170.
Feofilova, E. P., Ivashechkin, A. A., Alekhin, A. I., & Sergeeva, Y. E. (2012). Fungal spores: dormancy, germination, chemical composition, and role in biotechnology. Applied Biochemistry and Microbiology, 48, 1–11.
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., & Gurr, S. J. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature, 484, 186–194.
Fitt, B. D. L., Brun, H., Barbetti, M. J., & Rimmer, S. R. (2006). World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 3–15.
Freimoser, F. M., Jakob, C. A., Aebi, M., & Tuor, U. (1999). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Applied and Environmental Microbiology, 65, 3727–3729.
Fries, N. (1984). Spore germination in the higher Basidiomycetes. Proceedings: Plant Sciences, 93, 205–222.
Gloeckner, H., Jonuleit, T., & Lemke, H.-D. (2001). Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar blue™. Journal of Immunological Methods, 252, 131–138.
Goegan, P., Johnson, G., & Vincent, R. (1995). Effects of serum protein and colloid on the Alamar blue assay in cell cultures. Toxicology In Vitro, 9, 257–266.
Gottlieb, D. (1950). The physiology of spore germination in fungi. Botanical Review, 16, 229–257.
Grgurinovic, C. A., Walsh, D., & Macbeth, F. (2006). Eucalyptus rust caused by Puccinia psidii and the threat it poses to Australia. EPPO Bulletin, 36, 486–489.
Hill, J. L., Hocking, A. D., & Whitfield, F. B. (1995). The role of fungi in the production of chloroanisoles in general purpose freight containers. Food Chemistry, 54, 161–166.
Holliday, J. L., Jones, S. A., Simpson, J. A., Glen, M., Edwards, J., Robinson, A., & Burgman, M. A. (2013). A novel spore collection device for sampling exposure pathways: a case study of Puccinia psidii. Plant Disease, 97, 828–834.
Hovmøller, M. S., Yahyaoui, A. H., Milus, E. A., & Justesen, A. F. (2008). Rapid global spread of two aggressive strains of a wheat rust fungus. Molecular Ecology, 17, 3818–3826.
Huang, Y. J., Fitt, B. D. L., & Hall, A. M. (2003). Survival of A-group and B-group Leptosphaeria maculans (phoma stem canker) ascospores in air and mycelium on oilseed rape stem debris. Annals of Applied Biology, 143, 359–369.
Jackson, S. L., & Bayliss, K. L. (2011). Spore traps need improvement to fulfil plant biosecurity requirements. Plant Pathology, 60, 801–810.
Johnson, R. D., & Lewis, B. G. (1994). Variation in host range, systemic infection and epidemiology of Leptosphaeria maculans. Plant Pathology, 43, 269–277.
Khalifa, R. A., Nasser, M. S., Gomaa, A. A., Osman, N. M., & Salem, H. M. (2013). Resazurin microtiter assay plate method for detection of susceptibility of multidrug resistant Mycobacterium tuberculosis to second-line anti-tuberculous drugs. Egyptian Journal of Chest Diseases and Tuberculosis, 62, 241–247.
Khangura, R., Speijers, J., Barbetti, M. J., Salam, M. U., & Diggle, A. J. (2007). Epidemiology of blackleg (Leptosphaeria maculans) of canola (Brassica napus) in relation to maturation of pseudothecia and discharge of ascospores in Western Australia. Phytopathology, 97, 1011–1021.
Legras, J. L., Merdinoglu, D., Cornuet, J., & Karst, F. (2007). Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Molecular Ecology, 16, 2091–2102.
Li, H., Sivasithamparam, K., & Barbetti, M. J. (2004). Germination and invasion by ascospores and pycnidiospores of Leptosphaeria maculans on spring-type Brassica napus canola varieties with varying susceptibility to blackleg. Journal of General Plant Pathology, 70, 261–269.
Li, H., Sivasithamparam, K., & Barbetti, M. J. (2006). Evidence supporting the polycyclic natures of blackleg disease (Leptosphaeria maculans) of oilseed rape in Australia and implications for disease management. Brassica, 8, 65–69.
Li, H., Sivasithamparam, K., & Barbetti, M. J. (2007). Soilborne ascospores and pycnidiospores of Leptosphaeria maculans can contribute significantly to blackleg disease epidemiology in oilseed rape ( Brassica napus ) in Western Australia. Australasian Plant Pathology, 36, 439–444.
Lim, K. T., Zahari, Z., Amanah, A., Zainuddin, Z., & Adenan, M. I. (2016). Development of resazurin-based assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei rhodesiense strain STIB 900 for the identification of potential anti-trypanosomal agents. Experimental Parasitology, 162, 49–56.
Martin, A., Camacho, M., Portaels, F., & Palomino, J. C. (2003). Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrobial Agents and Chemotherapy, 47, 3616–3619.
Mascotti, K., McCullough, J., & Burger, S. R. (2000). HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion, 40, 693–696.
McNeill, M., Phillips, C., Young, S., Shah, F., Aalders, L., Bell, N., Gerard, E., & Littlejohn, R. (2011). Transportation of nonindigenous species via soil on international aircraft passengers’ footwear. Biological Invasions, 13, 2799–2815.
Miki, A., Isogai, A., Utsunomiya, H., & Iwata, H. (2005). Identification of 2,4,6-trichloroanisole (TCA) causing a musty/muddy off-flavor in sake and its production in rice koji and Moromi mash. Journal of Bioscience and Bioengineering, 100, 178-183.
Munshi, S., Twining, R. C., & Dahl, R. (2014). Alamar blue reagent interacts with cell-culture media giving different fluorescence over time: potential for false positives. Journal of Pharmacological and Toxicological Methods, 70, 195–198.
Murray, G. M., & Brennan, J. P. (2009). Estimating disease losses to the Australian wheat industry. Australasian Plant Pathology, 38, 558–570.
Murray, G. M., & Brennan, J. P. (2010). Estimating disease losses to the Australian barley industry. Australasian Plant Pathology, 39, 85–96.
Murray, G. M., & Brennan, J. P. (2012). The current and potential costs from diseases of oilseed crops in Australia. In GRDC (Ed.), (pp. 1–92): Grain Research Development Corporation.
Nakayama, G. R., Caton, M. C., Nova, M. P., & Parandoosh, Z. (1997). Assessment of the Alamar blue assay for cellular growth and viability in vitro. Journal of Immunological Methods, 204, 205–208.
O’Brien, J., Wilson, I., Orton, T., & Pognan, F. (2000). Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry, 267, 5421–5426.
Page, B., Page, M., & Noel, C. (1993). A new fluorometric assay for cytotoxicity measurements in-vitro. International Journal of Oncology, 3, 473–476.
Punithalingam, E., & Holliday, P. (1972). Leptosphaeria Maculans. IMI descriptions of fungi and bacteria (pp. sheet 331). Wallingford, UK: CAB International.
Rakotonirainy, M. S., Heraud, C., & Lavédrine, B. (2003). Detection of viable fungal spores contaminant on documents and rapid control of the effectiveness of an ethylene oxide disinfection using ATP assay. Luminescence, 18, 113–121.
Rampersad, S. N. (2012). Multiple applications of Alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors, 12, 12347–12360.
Riss, T. L., Moravec, R. A., Niles, A. L., Benink, H. A., Worzella, T. J., & Minor, L. (2013). Cell viability assays. Assay Guidance Manual (pp. 1–27).
Sarker, S. D., Nahar, L., & Kumarasamy, Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods, 42, 321–324.
Savage, D., Barbetti, M. J., Macleod, W. J., Salam, M. U., & Renton, M. (2012). Seasonal and diurnal patterns of spore release can significantly affect the proportion of spores expected to undergo long-distance dispersal. Microbial Ecology, 63, 578–585.
Savage, D., Barbetti, M. J., MacLeod, W. J., Salam, M. U., & Renton, M. (2013). Temporal patterns of ascospore release in Leptosphaeria maculans vary depending on geographic region and time of observation. Microbial Ecology, 65, 584–592.
Savary, S., Ficke, A., Aubertot, J.-N., & Hollier, C. (2012). Crop losses due to diseases and their implications for global food production losses and food security. Food Security, 4, 519–537.
Sterflinger, K. (2010). Fungi: their role in deterioration of cultural heritage. Fungal Biology Reviews, 24, 47–55.
Strange, R. N., & Scott, P. R. (2005). Plant disease: a threat to global food security. Annual Review of Phytopathology, 43, 83–116.
Strober, W. (2001). Trypan blue exclusion test of cell viability. Current protocols in immunology, A3-B:doi:10.1002/0471142735.ima03bs21.
Sturaro, A., Rella, R., Parvoli, G., Ferrara, D., & Tisato, F. (2006). Contamination of dry foods with trimethyldiphenylmethanes by migration from recycled paper and board packaging. Food Additives & Contaminants, 23, 431–436.
Tan, D. C., Flematti, G. R., Ghisalberti, E. L., Sivasithamparam, K., Chakraborty, S., Obanor, F., Jayasena, K., & Barbetti, M. J. (2012). Mycotoxins produced by Fusarium spp. associated with Fusarium head blight of wheat in Western Australia. Mycotoxin Research, 28, 89–96.
Tiballi, R. N., He, X., Zarins, L. T., Revankar, S. G., & Kauffman, C. A. (1995). Use of a colorimetric system for yeast susceptibility testing. Journal of Clinical Microbiology, 33, 915–917.
Tindale, C. R., Whitfield, F. B., Levingston, S. D., & Nguyen, T. H. L. (1989). Fungi isolated from packaging materials: their role in the production of 2, 4, 6-trichloroanisole. Journal of the Science of Food and Agriculture, 49, 437–447.
Twigg, R. S. (1945). Oxidation-reduction aspects of resazurin. Nature, 155, 401–402.
Wellings, C. R. (2007). Puccinia striiformis in Australia: a review of the incursion, evolution and adaptation of stripe rust in the period 1979–2006. Australian Journal of Agricultural Research, 58, 567–575.
Wellings, C. R. (2011). Global status of stripe rust: a review of historical and current threats. Euphytica, 179, 129–141.
West, J. S., & Fitt, B. D. L. (2005). Population dynamics and dispersal of Leptosphaeria maculans (blackleg of canola): adapted from a keynote address at the 15th biennial conference of the Australasian plant pathology society, 26-29 September 2005, Geelong. Australasian Plant Pathology, 34, 457–461.
West, J. S., Biddulph, J. E., Fitt, B. D. L., & Gladders, P. (1999). Epidemiology of Leptosphaeria maculans in relation to forecasting stem canker severity on winter oilseed rape in the UK. Annals of Applied Biology, 135, 535–546.
West, J. S., Kharbanda, P. D., Barbetti, M. J., & Fitt, B. D. L. (2001). Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology, 50, 10–27.
Willetts, H. J. (1971). The survival of fungal sclerotia under adverse environmental conditions. Biological Reviews, 46, 387–407.
Wilson, R. A., & Talbot, N. J. (2009). Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nature Reviews Microbiology, 7, 185–195.
Wyatt, T. T., Wösten, H. A., & Dijksterhuis, J. (2013). Fungal spores for dispersion in space and time. Advances in Applied Microbiology, 85, 43–91.
Xiao, J., Zhang, Y., Wang, J., Yu, W., Wang, W., & Ma, X. (2010). Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Applied Biochemistry and Biotechnology, 162, 1996–2007.
Zain, M. E. (2011). Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society, 15, 129–144.
Acknowledgments
We greatly appreciate the scholarship and operating funding provided by the Plant Biosecurity CRC for project CRC62042: ‘Curtailing and managing exotic fungal spore incursion into Australia’, and financial support by School of Plant Biology at the University of Western Australia. We thank Mr. Robert Creasy and Dr. Michael Considine from the University of Western Australia for the use of facilities and technical help during these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barua, P., You, M.P., Bayliss, K. et al. A rapid and miniaturized system using Alamar blue to assess fungal spore viability: implications for biosecurity. Eur J Plant Pathol 148, 139–150 (2017). https://doi.org/10.1007/s10658-016-1077-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1077-5