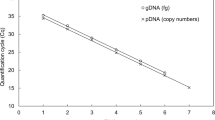
Abstract
Apple scab caused by the fungus Venturia inaequalis can result in significant crop losses if not managed effectively. Sanitation as part of an integrated management strategy aims to significantly reduce primary inoculum to lower the disease pressure. This study evaluates the possibility of molecular detection and quantification of ascospore discharge and the use of this method to test for efficacy of orchard sanitation treatments. A method to detect and quantify airborne ascospores was developed using volumetric spore traps (VSTs). V. inaequalis specific primers were tested on daily VST samples from two orchard sections (leaf litter removed compared to leaf litter left) during spring. A molecular method to detect and quantify ascospores was tested by amplifying genomic regions of the mitochondrial CYP51A1 gene, and the ITS region using SYBR® green. Timing of ascospore discharge was compared to predicted infection risk and a degree day model using weather data. The average spore detection limit was estimated to be at levels of 1 pg μl−1 DNA (approximately 37 ascospores) per daily spore trap reading using CYP51A1 primers. Using the CYP51A1 primer pair, primary inoculum was estimated to be 51 % lower in the orchard sections where leaves had been removed, indicating that this method could be used to evaluate the efficacy of alternative control strategies such as leaf removal to reduce potential ascospore dose. This is the first report of combining VSTs and quantitative PCR to monitor airborne V. inaequalis ascospores.




Similar content being viewed by others
References
Aylor, D. E. (1993). Relative collection efficiency of rotorod and burkard spore samplers for airborne venturia inaequalis ascospores. Phytopathology, 83, 1116–1119.
Burchill, R. T., & Hutton, K. E. (1965). The suppression of ascospore production to facilitate the control of apple scab (Venturia inaequalis [Cke.] Wint.). Annals of Applied Biology, 56, 285–292.
Burchill, R. T. (1968). Field and laboratory studies of the effect of urea on ascospore production of Venturia inaequalis (Cke.) Wint. Annals of Applied Biology, 62, 297–307.
Carisse, O., McCartney, H. A., Gagnon, J. A., & Brodeur, L. (2005). Quantification of airborne inoculum as an aid in the management of leaf blight of onion caused by Botrytis squamosa. Plant Disease, 89, 726–733.
Carisse, O., Rolland, D., Talbot, B., & Savary, S. (2007). Heterogeneity of the aerial concentration and deposition of ascospores of Venturia inaequalis within a tree canopy during the rain. European Journal of Plant Pathology, 117, 13–24.
Carisse, O., Jobin, T., & Bourgeois, G. (2008). Predicting apple leaf emergence from degree-day accumulation during the primary scab period. Canadian Journal of Plant Science, 88, 229–238.
Carisse, O., Tremblay, D. M., Levesque, C. A., Gindro, K., Ward, P., & Houde, A. (2009). Development of a TaqMan Real-Time PCR assay for quantification of airborne conidia of Botrytis squamosa and management of Botrytis leaf blight of onion. Phytopathology, 99, 1273–1280.
Carisse, O., Tremblay, D.-M., Jobin, T., & Walker, A. S. (2011). Disease decision support systems: their impact on disease management and durability of fungicide effectiveness. In O. Carisse (Ed.), Fungicides. InTech: Publishing.
Charest, J., Dewdney, M., Paulitz, T., Philion, V., & Carisse, O. (2002). Spatial distribution of Venturia inaequalis airborne ascospores in orchards. Phytopathology, 92, 769–779.
Creemers, P., Vanmechelen, A., & Hauke, K. (2006). Bulletin OILB/SROP. Dijon France: International Organization for Biological and Integrated Control of Noxious Animals and Plants. Sanitation practices to reduce apple scab inoculum in orchards.
Daniëls, B., De Landtsheer, A., Dreesen, R., Davey, M. W., & Keulemans, J. (2012). Real-time PCR as a promising tool to monitor growth of Venturia spp. in scab-susceptible and -resistant apple leaves. European Journal of Plant Pathology, 134, 821–833.
Diguta, C. F., Rousseaux, S., Weidmann, S., Bretin, N., Vincent, B., Guilloux-Benatier, M., & Alexandre, H. (2010). Development of a qPCR assay for specific quantification of Botrytis cinerea on grapes. Fems Microbiology Letters, 313, 81–87.
Gadoury, D. M., & MacHardy, W. E. (1982). A model to estimate the maturity of ascospores of Venturia inaequalis. Phytopathology, 72, 901–904.
Gadoury, D. M., & Machardy, W. E. (1986). Forecasting ascospore dose of Venturia inaequalis in commercial apple orchards. Phytopathology, 76, 112–118.
Gadoury, D. M., Stensvand, A., & Seem, R. C. (1998). Influence of light, relative humidity, and maturity of populations on discharge of ascospores of Venturia inaequalis. Phytopathology, 88, 902–909.
Gadoury, D. M., Seem, R. C., & Machardy, W. E. (2004). A Comparison of methods used to estimate the maturity and release of ascospores of Venturia inaequalis. Plant Disease, 88, 869–874.
Gehesquière, B., D’Haeyer, S., Pham, K. T. K., Van Kuik, A. J., Maes, M., Hoefte, M., & Heungens, K. (2013). QPCR assays for the detection of Cylindrocladium buxicola in plant, water, and air samples. Plant Disease, 97, 1082–1090.
Giosue, S., Rossi, V., Ponti, I., & Bugiani, R. (2000). Estimating the dynamics of airborne ascospores of Venturia inaequalis. Bulletin OEPP/EPPO Bulletin, 30, 137–142.
Gusberti, M., Patocchi, A., Gessler, C., & Broggini, G. A. L. (2012). Quantification of Venturia inaequalis growth in Malus x domestica with quantitative real-time polymerase chain reaction. Plant Disease, 96, 1791–1797.
Hirst, J. M., & Stedman, O. J. (1961). The epidemiology of apple scab (Venturia inaequalis [Cke.] Wint.) I. Frequency of airborne spores in the orchard. Annals of Applied Biology, 49, 290–305.
Holb, I. J. (2006). Effect of six sanitation treatments on leaf litter density, ascospore production of Venturia inaequalis and scab incidence in integrated and organic apple orchards. European Journal of Plant Pathology, 115, 293–307.
Holb, I. J. (2007). Effect of four non-chemical sanitation treatments on leaf infection by Venturia inaequalis in organic apple orchards. European Journal of Horticultural Science, 72, 60–65.
Holb, I. J. (2008). Timing of first and final sprays against apple scab combined with leaf removal and pruning in organic apple production. Crop Protection, 27, 814–822.
James, J. R., & Sutton, T. B. (1982). A model for predicting ascospore maturation of Venturia inaequalis. Phytopathology, 72, 1081–1085.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierier, T., Ashton, B., Meintjes, P., & Drummond, A. J. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649.
Kupfer, D. M., Reece, C. A., Clifton, S. W., Roe, B. A., & Prade, R. A. (1997). Multicellular ascomycetous fungal genomes contain more than 8000 genes. Fungal Genetics and Biology, 21, 364–372.
Lu, Q. F., Hu, H. Q., Mo, J. J., & Shu, L. Z. (2012). Enhanced amplification of bacterial and fungal DNA using a new type of DNA polymerase. Australasian Plant Pathology, 41, 661–663.
Luo, Y., Ma, Z., Reyes, H. C., Morgan, D., & Michailides, T. J. (2007). Quantification of airborne spores of Monilinia fructicola in stone fruit orchards of California using real-time PCR. European Journal of Plant Pathology, 118, 145–154.
Ma, Z. H., & Michailides, T. J. (2007). Approaches for eliminating PCR inhibitors and designing PCR primers for the detection of phytopathogenic fungi. Crop Protection, 26, 145–161.
MacHardy, W. E., Gadoury, D. M., & Rosenberger, D. A. (1993). Delaying the onset of fungicide programs for control of apple scab in orchards with low potential ascospore dose of Venturia inaequalis. Plant Disease, 77, 372–375.
MacHardy, W. E. (1994). A “PAD” action threshold: The key to integrating practices for managing apple scab. Norwegian Journal of Agricultural Sciences, Supplement, 17, 75–82.
MacHardy, W.E. (1996). Models to predict ascospore maturity. In W.E. MacHardy (Ed.), Apple scab: biology, epidemiology, and management. St. Paul, Minnesota USA: American Phytopathological Society (APS Press): 251 pp.
Mills, W. D., & Laplante, A. A. (1951). Diseases and insects in the orchard. Cornell Extension Bulletin, 711, 20–27.
Mota, M., & Oliveira, C. (2005) Caracterização do DNA ribossomal do fungo Venturia inaequalis isolado em pomares de macieira em Portugal. Actas de Horticultura do V Congresso Ibérico de Ciências Hortícolas, IV Congresso Iberoamericano de Ciências Hortícolas, Porto, Maio de 2005, 137–143
Ostle, B. (1954). Correlation methods. In B. Ostle (Ed.), Statistics in research (pp. 174–201). Ames: Iowa State College Press.
Ribbert, M. (2004) Herstellung vom Antikörpern gegen Venturia inaequalis zur Entwicklung von Biosensoren. http://darwin.bth.rwth-aachen.de/opus3/volltexte/2004/828/ thesis downloaded 13.03.2013
Ribbert, M. (2005). Immunodetection of Venturia inaequalis ascospores with phage antibodies. Proceedings of the 7th International IOBC/WPRS workshop on orchard diseases, p.6
Rossi, V., Ponti, I., Marinelli, M., Giosue, S., & Bugiani, R. (2001). Environmental factors influencing the dispersal of Venturia inaequalis ascospores in the orchard air. Journal of Phytopathology-Phytopathologische Zeitschrift, 149, 11–19.
Rossi, V., Giosue, S., & Bugiani, R. (2007). A-scab (Apple-scab), a simulation model for estimating risk of Venturia inaequalis primary infections. Bulletin OEPP/EPPO Bulletin, 37, 300–308.
Rogers, S. L., Atkins, S. D., & West, J. S. (2009). Detection and quantification of airborne inoculum of Sclerotinia sclerotiorum using quantitative PCR. Plant Pathology, 58, 324–331.
Schnabel, G., & Jones, A. L. (2001). The 14 alpha-demethylase (CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology, 91, 102–110.
Schoch, C. L., Crous, P. W., Groenewald, J. Z., Boehm, E. W. A., Burgess, T. I., de Gruyter, J., de Hoog, G. S., Dixon, L. J., Grube, M., Gueidan, C., Harada, Y., Hatakeyama, S., Hirayama, K., Hosoya, T., Huhndorf, S. M., Hyde, K. D., Jones, E. B. G., Kohlmeyer, J., Kruys, A., Li, Y. M., Lucking, R., Lumbsch, H. T., Marvanova, L., Mbatchou, J. S., McVay, A. H., Miller, A. N., Mugambi, G. K., Muggia, L., Nelsen, M. P., Nelson, P., Owensby, C. A., Phillips, A. J. L., Phongpaichit, S., Pointing, S. B., Pujade-Renaud, V., Raja, H. A., Plata, E. R., Robbertse, B., Ruibal, C., Sakayaroj, J., Sano, T., Selbmann, L., Shearer, C. A., Shirouzu, T., Slippers, B., Suetrong, S., Tanaka, K., Volkmann-Kohlmeyer, B., Wingfield, M. J., Wood, A. R., Woudenberg, J. H. C., Yonezawa, H., Zhang, Y., & Spatafora, J. W. (2009). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology, 64, 1–15.
Schwabe, W. F. S. (1977). Tolerance of Venturia inaequalis to benzimidazole fungicides and dodine in South Africa. Phytophylactica, 9, 47–54.
Schwabe, W. F. S. (1980a). Wetting and temperature requirements for apple leaf infection by Venturia inaequalis in South Africa. Phytophylactica, 12, 69–80.
Schwabe, W. F. S. (1980b). Curative activity of fungicides against apple leaf infection by Venturia inaequalis. Phytophylactica, 12, 199–207.
Schwabe, W. F. S., Jones, A. L., & van Blerk, E. (1989). Relation of degree-day accumulations to maturation of ascospores of Venturia inaequalis in South Africa. Phytophylactica, 21, 12–16.
Sholberg, P., O’Gormann, D., Bedford, K., & Lévesque, C. A. (2005). Development of a DNA macroarray for detection and monitoring of economically important apple diseases. Plant Disease, 89, 1143–1150.
Stehmann, C., Pennycook, S., & Plummer, K. M. (2001). Molecular identification of a sexual interloper: The pear pathogen, Venturia pirina, has sex on apple. Phytopathology, 91, 633–641.
Stensvand, A., Eikemo, H., Gadoury, D. M., & Seem, R. C. (2005). Use of a rainfall frequency threshold to adjust a degree-day model of ascospore maturity of Venturia inaequalis. Plant Disease, 89, 198–202.
Sutton, D. K., MacHardy, W. E., & Lord, W. G. (2000). Effects of shredding or treating apple leaf litter with urea on ascospore dose of Venturia inaequalis and disease buildup. Plant Disease, 84, 1319–1326.
Von Diest, S., Meitz, J., & Lennox, C. (2011). Orchard sanitation as a management approach to control apple scab in South African apple orchards. IOBC bulletin, 84, 331–336.
Warner, J., & Braun, P. G. (1992). Discharge of Venturia inaequalis ascospores during daytime and nighttime wetting periods in Ontario and Nova-Scotia. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 14, 315–321.
West, J. S., Atkins, S. D., Emberlin, J., & Fitt, B. D. L. (2008). PCR to predict risk of airborne disease. Trends in Microbiology, 16, 380–387.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols: A Guide to Methods and Applications (pp. 315–322). New York: Academic.
Williams, R. H., Ward, E., & McCartney, H. A. (2001). Methods for integrated air sampling and DNA analysis for detection of fungal spores. Applied Environmental Microbiology, 67, 2453–2459.
Zeng, Q. Y., Westermark, S. O., Rasmuson-Lestander, A., & Wang, X. R. (2006). Detection and quantification of Cladosporium in aerosols by real-time PCR. Journal of Environmental Monitoring, 8, 153–160.
Zhang, Z., Kermekchiev, M. B., & Barnes, W. M. (2010). Direct DNA amplification from crude clinical samples using a PCR enhancer cocktail and novel mutants of Taq. Journal of Molecular Diagnostics, 12, 152–161.
Zuck, M. G., & Caruso, F. L. (1984). A volumetric spore trap designed for monitoring Venturia inaequalis spore release in apple scab management programs. Phytopathology, 74, 796.
Acknowledgments
This work was supported through Hortgro Science (formerly known as deciduous fruit producer’s trust) for project- and MSc bursary funding, the National Research Foundation for project funding (THRIP, Y-rated), and the Claude Leon foundation for postdoc bursary funding. We would like to express our gratitude to Dr. Wolf Schwabe for his lifetime work on apple scab and his mentoring for this project. Thanks also to Prof. Martin Kidd for statistical data analysis. We also would like to thank the South African fruit growers and Bekker Wessels for their participation in this study and Armandt Le Roux and Jessica Rochefort for expert technical assistance in the molecular lab.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Spore number estimates and qPCR melting temperature [TM] of samples collected by VST (leaf removal [LR] or negaitve control [C] orchard sections) and quantified using qPCR (PDF 404 kb)
Rights and permissions
About this article
Cite this article
Meitz-Hopkins, J.C., von Diest, S.G., Koopman, T.A. et al. A method to monitor airborne Venturia inaequalis ascospores using volumetric spore traps and quantitative PCR. Eur J Plant Pathol 140, 527–541 (2014). https://doi.org/10.1007/s10658-014-0486-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0486-6