Abstract
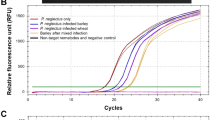
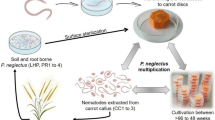
The polyphagous obligate parasites Meloidogyne spp. devastate a wide range of crop plants including bananas and plantains. Their infestations impact agriculture worldwide. Therefore, an effective combating regime against this nematode species and an in-depth understanding of plant-nematode interaction are essential. Early detection of infection by visual inspection is not possible. This hampers early control strategy efforts and makes in-depth research of the early infection and plant defence unfeasible. A simple and robust in planta PCR-based nematode detection method is described here as the first crucial step. This PCR-based detection assay exploits the existence of the Internal Transcribed Spacer 1 (ITS 1) region of the ribosomal DNA (rDNA) gene family in the nematodes for early detection of nematode penetration into the roots. The results demonstrate that this detection assay is suitable to serve as a molecular screening tool for plant root diagnostic purposes.





Similar content being viewed by others
Abbreviations
- IP:
-
Infected plant root
- N:
-
Nematode individual
- ND:
-
Nematode DNA
- NP:
-
Nematode mixed with plant roots
- NPD:
-
Nematode and plant DNA
- P:
-
Plant roots
- PD:
-
Plant DNA
- TR:
-
Transformed tomato roots infected with Meloidogyne incognita
References
Atkins, S. D., Clark, I. M., Penny, S.-P., Hirsch, R., & Kerry, B. R. (2005). The use of real-time PCR and species-specific primers for the identification and monitoring of Paecilomyces lilacinus. FEMS Microbiology Ecology, 51, 257–264.
Brody, J. R., & Kern, S. E. (2004). History and principles of conductive media for standard DNA electrophoresis. Analytical Biochemistry, 333, 1–13.
Cold Spring Harbour Protocols. (2009). Cetyltrimethyl Ammonium Bromide (CTAB) DNA Miniprep for Plant DNA Isolation. doi:10.1101/pdb.prot5177. Retrieved from http://cshprotocols.cshlp.org/cgi/content/abstract/2009/3/pdb.prot5177.
Davide, R. G., & Marasigan, L. Q. (1985). Yield loss assessment and evaluation of resistance of banana cultivars to the nematodes Radopholus similis Thorne and Meloidogyne incognita Chitwood. Philippine Agriculturist, 68, 335–349.
De Schutter, B., Speijer, P. R., Dochez, C., Tenkouano, A., & De Waele, D. (2001). Evaluating host plant reaction of Musa germplasm to Radopholus similis by inoculation of single primary roots. Nematropica, 31, 295–299.
De Waele, D., & Davide, R. G. (1998). The root-knot nematodes of banana: M. incognita and M. javanica. INIBAP Musa Pest Fact sheet No. 3.
Dellaporta, S. L., Wood, J., & Hicks, J. B. (1983). A plant DNA minipreparation: Version II. Plant Molecular Biology Reporter, 4, 19–21.
Ding, L.-W., Sun, Q.-Y., Wang, Z.-Y., Sun, Y.-B., & Xu, Z.-F. (2008). Using silica particles to isolate total RNA from plant tissues recalcitrant to extraction in guanidine thiocyanate. Analytical Biochemistry, 374, 426–428.
Fallas, G. A., Hahn, M. L., Fargette, M., Burrows, P. R., & Sarah, J. L. (1996). Molecular and biochemical diversity among isolates of Radopholus spp. from different areas of the world. Journal of Nematology, 28, 422–430.
Frison, E. A., & Sharrock, S. (1998). The economic, social and nutritional importance of banana in the world. In C. Picq, E. Fouré, & E. A. Frison (Eds.), Bananas and food security Proceedings of an international symposium held in Douala (pp. 21–35). INIBAP: Cameroon. France.
Gheysen, G., & Jones, J. T. (2006). Molecular aspects of plant-nematode interactions. In R. N. Perry & M. Moens (Eds.), Plant Nematology (pp. 234–254). Wallingford: CABI Publishing.
Gowen, S. R., Quénéhervé, P., & Fogain, R. (2005). Nematode parasites of bananas and plantains. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), In Plant parasitic nematodes in subtropical and tropical agriculture (pp. 611–643). Wallingford: CABI Publishing.
Hirsch, P. R., Mauchline, T. H., Mendum, T. A., & Kerry, B. R. (2000). Detection of the nematophagous fungus Verticillium chlamydosporium in nematode-infested plant roots using PCR. Mycological Research, 104, 435–439.
Hubschen, J., Kling, L., Ipach, U., Zinkernagel, V., Brown, D., & Neilson, R. (2004). Development and validation of species-specific primers that provide a molecular diagnostic for virus-vector longidorid nematodes and related species in German viticulture. European Journal of Plant Pathology, 110, 883–891.
Jones, D. R. (2000). Diseases of banana, Abacá and Ensete. Wallingford: CABI Publishing.
Khayat, E., Duvdevani, A., Lahav, E., & Balesteros, B. A. (2004). Somaclonal variation in banana (Musa acuminata cv. Grande Naine): genetic mechanism, frequency and application as a tool for clonal selection. In S. M. Jain & R. Swennen (Eds.), Banana improvement: cellular, molecular biology and induced mutation (pp. 97–109). Science Publishers Inc: Plymouth.
Lovic, B. R., Valadez, V. A., Martyn, R. D., & Miller, M. E. (1995). Detection and identification of Monosporascus spp with genus-specific PCR primers and nonradioactive hybridization probes. Plant Disease, 79, 1169–1175.
Madani, M., Subbotin, S. A., & Moens, M. (2005). Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using Real-Time PCR with SYBR green I dye. Molecular and Cellular Probes, 19, 81–86.
McCartney, H. A., Foster, S. J., Fraaije, B. A., & Ward, E. (2003). Molecular diagnostics for fungal plant pathogens. Pest Management Science, 59, 129–124.
McCuiston, J. L., Hudson, L. C., Subbotin, S. A., Davis, E. L., & Warfield, Y. C. (2007). Conventional and PCR detection of Aphelenchoides fragariae in diverse ornamental host plant species. Journal of Nematology, 39, 343–355.
Powers, T. O., Mullin, P. G., Harris, T. S., Sutton, L. A., & Higgins, R. S. (2005). Incorporating molecular identification of Meloidogyne spp. into a large-scale regional nematode survey. Journal of Nematology, 37, 226–235.
Qiu, J. J., Westerdahl, B. B., Anderson, C., & Williamson, V. M. (2006). Sensitive PCR detection of Meloidogyne arenaria, M. incognita and M. javanica extracted from soil. Journal of Nematology, 38, 434–441.
Razak, A. R. (1994). Plant parasitic nematodes, a potential threat to commercial cultivation of banana in Malaysia. In R. V. Valmayor, R. G. Davide, J. M. Stanton, N. L. Treverrow, & V. N. Roa (Eds.), Banana nematodes and weevil borers in Asia and the Pacific. Proceedings of a conference-workshop on nematodes and weevil borers affecting bananas in Asia and the Pacific, Serdang, Selangor, Malaysia (pp. 34–45). Los Baños: INIBAP/ASPNET.
Rowhani, A., Chay, C., Golino, D. A., & Falk, B. (1993). Development of a polymerase chain reaction technique for the detection of grapevine fanleaf virus in grapevine tissue. Phytophatology, 83, 749–753.
Subbotin, S. A., & Moens, M. (2006). Molecular taxonomy and phylogeny. In R. N. Perry & M. Moens (Eds.), Plant nematology (pp. 33–58). Wallingford: CABI.
Sundelin, T., Collinge, D. B., & Lubeck, M. (2009). A cultivation independent, PCR-based protocol for the direct identification of plant pathogens in infected plant material. European Journal of Plant Pathology, 123, 473–476.
Thomson, D., & Dietzgen, R. G. (1995). Detection of DNA and RNA plant viruses by PCR and RT-PCR using a rapid virus release protocol without tissue homogenisation. Journal of Virological Methods, 54, 85–95.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882.
Williamson, V. M., & Gleason, C. A. (2003). Plant–nematode interactions. Current Opinion in Plant Biology, 6, 327–333.
Williamson, V., Caswell-Chen, E., Westerdahl, B., Wu, F., & Caryl, G. (1997). A PCR assay to identify and distinguish single juveniles of Meloidogyne hapla and M. chitwoodi. Journal of Nematology, 29, 9–15.
Wilson, I. G. (1997). Miniriview: Inhibition and facilitation of nucleic acid amplification. Applied and Environmental Microbiology, 63, 3741–3751.
Yeates, C., Gillings, M. R., Davidson, A. D., Altavilla, N., & Veal, D. A. (1998). Methods for microbial DNA extraction from soil for PCR amplification. Biological Procedures Online, 1, 40–47.
Zijsltra, C., van Hoof, R., & Donkers-Venne, D. (2004). A PCR test to detect the cereal root-knot nematode Meloidogyne naasi. European Journal of Plant Pathology, 110, 855–860.
Zouhar, M., Marek, M., Douda, O., Mazáková, J., & Ryšánek, P. (2007). Conversion of sequence-characterized amplified region (SCAR) bands into high throughput DNA markers based on RAPD technique for detection of the stem nematode Ditylenchus dipsaci in crucial plant hosts. Plant Soil and Environment, 53, 97–104.
Acknowledgement
This work was supported by the Ministry of Science, Technology and Innovation Malaysia, through the National e-Science Fund 02-01-03-SF0088. We would like to acknowledge University of Malaya, Kuala Lumpur, Malaysia for funding the first author’s stay in the Laboratory of Tropical Crop Improvement, Leuven, Belgium. We also wish to thank Annemie Elsen, Christine Vos, Els Thiry and Isabelle Henry for their continuous assistance and valuable comments during this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sayed Abdul Rahman, S.A., Mohamed, Z., Othman, R.Y. et al. In planta PCR-based detection of early infection of plant-parasitic nematodes in the roots: a step towards the understanding of infection and plant defence. Eur J Plant Pathol 128, 343–351 (2010). https://doi.org/10.1007/s10658-010-9656-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-010-9656-3