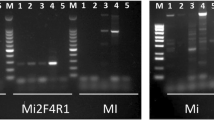
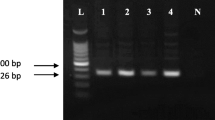
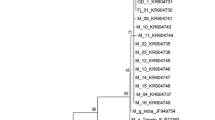
Abstract
The nematode species Longidorus attenuatus, L. elongatus, L. macrosoma and Paralongidorus maximusare economically important pests to the viticulture industry due to their ability to vector two nepoviruses (Raspberry Ringspot Virus and Tomato Black Ring Virus) to grapevines. In Germany, these species occur in vineyard soil with other non-vector but morphologically similar longidorid species, L. helveticus, L.␣profundorum and L. sturhani. Species-specific primers were designed from ribosomal DNA for all seven species to facilitate taxonomic identification for non-specialists. Primers were assessed for their reliability by screening, where possible, a number of populations of each species. Furthermore, their selectivity and sensitivity were determined when challenged with closely related longidorid species and general nematode communities typical of vineyard soil. A multiplex approach using a common forward primer combined with species-specific reverse primers enabled three target nematode species to be detected in the same PCR reaction. All primers were highly specific, detecting all nematode developmental forms from disparate populations and were sufficiently sensitive to detect a single target nematode within a whole nematode community typical of a vineyard soil comprising of a range of non-target species. Given their specificity, sensitivity and reliability, these diagnostic primers should be of great benefit to both phytosanitary/quarantine services related to the viticulture industry and also as a decision management tool for growers.
Similar content being viewed by others
References
Alphey TJW and Taylor CE (1986) European Atlas of the Longidoridae and Trichodoridae. Scottish Crop Research Institute, Dundee, UK
Amiri S, Subbotin SA and Moens M(2002) Identification of the beet cyst nematode Heterodera schachtii by PCR. European Journal of Plant Pathology 108: 497-506
Boutsika K, Phillips MS, MacFarlane SA, Brown DJF, Holeva RC and Blok VC (2004) Molecular diagnostics of some trichodorid nematodes and associated Tobacco rattle virus. Plant Pathology 53: 110-116
Brown DJF and Taylor CE (1987) Comments on the occur-rence and geographical distribution of longidorid nematodes in Europe and the mediterranean region. Nematologia mediterranea 15: 333-373
Brown DJF and Trudgill DL (1989) The occurrence and distribution of nepoviruses and their associated vector Longidorus and Xiphinema nematodes in Europe and the Mediterranean Basin. European Plant Protection Organisation Bulletin 19: 479-489
Chen QW, Hooper DJ, Loof PAA and Xu J (1997) A revised polytomous key for the identification of species of the genus Longidorus Micoletzky, 1922 (Nematoda: Dorylaimoidea). Fundamental and Applied Nematology 20: 15-28
Cherry T, Szalanski AL, Todd TC and Powers TO (1997) The internal transcribed spacer region of Belonolaimus (Nemata: Belonolaimidae). Journal of Nematology 29: 23-29
Dong K, Dean RA, Fortnum BA and Lewis SA (2001) A species-specific DNA probe for the identification of Meloi-dogyne hapla. Nematropica 31: 17-23
Fleming CC, Turner SJ, Powers TO and Szalanski AL (1998) Diagnostics of cyst nematodes: use of the polymerase chain reaction to determine species and estimate population levels. Aspects of Applied Biology 52: 375-382
Fritzsche R and Kegler H (1968) Nematoden als Vektoren von Viruskrankheiten der Obstgewa ¨chse. Tagungsberichte, Deut-sche Akademie der Landwirtschaftswissenschaften, Berlin 97: 289-295
Harrison BD (1964) Specific nematode vectors for serologically distinctive forms of raspberry ringspot and tomato black ring viruses. Virology 22: 544-550
Harrison BD, Mowat WP and Taylor CE (1961) Transmission of a strain of tomato black ring virus by Longidorus elongatus (Nematoda). Virology 14: 480-485
Hoyer U, Burgermeister W and Braasch H (1998) Identification of Bursaphelenchus species (Nematoda, Aphelenchoididae) on the basis of ampli ed ribosomal DNA (ITS-RFLP). Nachrichtenblatt des Deutschen P. anzenschutzdienstes 50: 273-277
Hu ¨bschen J, Kling L, Ipach U, Zinkernagel V, Bosselut N, Esmenjaud D, Brown DJF and Neilson R (in press) Validation of the specificity and sensitivity of species-specific primers that provide a reliable molecular diagnostic for Xiphinema diversicaudatum, X. index and X. vuittenezi. European Journal of Plant Pathology 110
Iwahori H, Tsuda K, Kanzaki N, Izui K and Futai K (1998) PCR-RFLP and sequencing analysis of ribosomal DNA of Bursaphelenchus nematodes related to pine wilt disease. Fundamental and Applied Nematology 21: 655-666
Knoetze R, Burger JT and Meyer AJ (2000) Discrimination of some Xiphinema species from South Africa by rDNA-RFLP analysis. African Plant Protection 6: 25-30
Jones AT, Brown DJF, McGavin WJ, Ru ¨del M and Altmayer B (1994) Properties of an unusual isolate of raspberry ringspot virus from grapevine in Germany and evidence for its possible transmission by Paralongidorus maximus. Annals of Applied Biology 124: 283-300
Lamberti F, Kunz P, Grunder J, Molinari S, De Luca F, Agostinelli A and Radicci V (2001) Molecular charcterisation of six Longidorus species from Switzerland with the description of Longidorus helveticus sp. n. (Nematoda, Dorylaimida). Nematologia mediterranea 29: 181-205
Liao JL, Zhang LH and Feng ZX (2001) Reliable identification of Bursaphelenchus xylophilus by rDNA ampli cation. Nematologia mediterranea 29: 131-135
Molinari S, De Luca F, Lamberti F and De Giorgi C (1997) Molecular methods for the identification of Longidorid nematodes. Nematologia mediterranea 25: 55-61
Mulholland V, Carde L, O 'Donnell KJ, Fleming CC and Powers TO (1996) Use of the polymerase chain reaction to discriminate potato cyst nematode at the species level. In: Marshall G (ed) BCPC Proceedings No. 65, Diagnostics in Crop Production (pp 247-252) BCPC, UK
Nguyen KB, Maruniak J and Adams BJ (2001) Diagnostic and phylogenetic utility of the rDNA internal transcribed spacer sequences of Steinernema. Journal of Nematology 33: 73-82
Powers TO, Todd TC, Burnell AM, Murray PCB, Fleming CC, Szalanski AL, Adams BA and Harris TS (1997) The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. Journal of Nematology 29: 441-450
Reid AP, Hominick WM and Briscoe BR (1997) Molecular taxonomy and phylogeny of entomopathogenic nematode species (Rhabditida: Steinernematidae) by RFLP analysis of the ITS region of the ribosomal DNA repeat unit. Systematic Parasitology 37: 187-193
Robbins RT, Brown DJF, Halbrendt JM and Vrain TC (1995) Compendium of Longidorus juvenile stages with observations on L. pisi, L. taniwha and L. diadecturus (Nematoda: Longidoridae). Systematic Parasitology 32: 33-52
Rubtsova TV, Subbotin SA, Brown DJF and Moens M (2001) Description of Longidorus sturhani sp. n. (Nematoda: Lon-gidoridae) and molecular characterisation of several longidorid species from Western Europe. Russian Journal of Nematology 9: 127-136
Ru ¨del M (1985) Grapevine damage induced by particular virus-vector combinations. Phytopathologia Mediterranea 24: 183-185
Sasser JN and Freckman DW (1987) A world perspective on nematology: the role of the society. In: Veech JA and Dickson DW (eds) Vistas on Nematology: A Commemoration of the Twenty-fifth Anniversary of the Society of Nematologists (pp 7-14).
Society of Nematologists, Maryland, USA Schaaf C (1999) Untersuchungen zur Wirkung von P. anzeninhaltssto. en und Feindp. anzen auf die phytopar-asita ¨ren Nematoden Xiphinema index Thorne & Allen 1950 und Meloidogyne incognita (Kofoid and White 1919) Chitwood 1949. Dissertation. Universita ¨t Hohenheim, Germany
Shields R, Fleming CC and Stratford R (1996) Identification of potato cyst nematodes using the polymerase chain reaction. Fundamental and Applied Nematology 19: 167-173
Stanton JM, McNicol CD and Steele V (1998) Non-manual lysis of second-stage Meloidogyne juveniles for identification of pure and mixed samples based on the polymerase chain reaction. Australasian Plant Pathology 27: 112-115
Stock SP, Campbell JF and Nadler SA (2001) Phylogeny of Steinernema Travassos 1927 (Cephalobina: Steinernemati-dae) inferred from ribosomal DNA sequences and morpho-logical characters. Journal of Parasitology 87: 877-889
Subbotin SA, Waeyenberge L and Moens M (2000) Identification of cyst forming nematodes of the genus Heterodera (Nematoda: Heteroderidae) based on the ribosomal DNA-RFLP. Nematology 2: 153-164
Taylor CE (1962) Transmission of raspberry ringspot virus by Longidorus elongatus (de Man) (Nematoda: Dorylaimidae). Virology 17: 493-494
Taylor CE and Brown DJF (1997) Nematode Vectors of Plant Viruses. CABI, Wallingford, UK
Trudgill DL, Brown DJF and McNamara DG (1983) Methods and criteria for assessing the transmission of plant viruses by longidorid nematodes. Revue de Ne ´matologie 6: 133-141
Uehara T, Mizukubo T, Kushida A and Momota Y (1998) Identification of Pratylenchus co. eae and P. loosi using specific primers for PCR ampli cation of ribosomal DNA. Nematologica 44: 357-368
Uehara T, Kushida A and Momota Y (1999) Rapid and sensitive identification of Pratylenchus spp. using reverse dot blot hybridization. Nematology 1: 549-555
Vrain TC (1993) Restriction fragment length polymorphism separates species of the Xiphinema americanum group. Journal of Nematology 25: 361-364
Vrain TC, Wakarchuk DA, Levesque AC and Hamilton RI (1992) Intraspecific rDNA restriction fragment length poly-morphism in the Xiphinema americanum group. Fundamental and Applied Nematology 15: 563-573
Waeyenberge L, Ryss A, Moens M, Pinochet J and Vrain TC (2000) Molecular characterisation of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology 2: 135-142
Wang X, Bosselut N, Castagnone C, Voisin R, Abad P and Esmenjaud D (2003) Multiplex polymerase chain reaction identification of single individuals of the Longidorid nema-todes Xiphinema index, X. diversicaudatum, X. vuittenezi, and X. italiae using specific primers from ribosomal genes. Phytopathology 93: 160-166
Wishart J, Phillips MS and Blok VC (2002) Ribosomal intergenic spacer: a polymerase chain reaction diagnostic for Meloidogyne chitwoodi, M. fallax and M. hapla. Phytopathology 92: 884-892
Yeates GW, Bongers T, de Geode RGM, Freckman DW and Georgieva SS (1993) Feeding habits in soil nematode families and genera-an outline for soil ecologists. Journal of Nematology 25: 315-331
Zheng J, Subbotin SA, Waeyenberge L and Moens M (2000) Molecular characterisation of Chinese Heterodera glycines and H. avenae populations based on RFLPs and sequences of rDNA-ITS regions. Russian Journal of Nematology 8: 109-113
Zijlstra C (1997) A fast PCR assay to identify Meloidogyne hapla, M. chitwoodi, and M. fallax, and to sensitively di. erentiate them from each other and from M. incognita in mixtures. Fundamental and Applied Nematology 20: 505-511
Zijlstra C (2000) Identification of Meloidogyne chitwoodi, M. fallax and M. hapla based on SCAR-PCR: a powerful way of enabling reliable identification of populations or individuals that share common traits. European Journal of Plant Pathology 106: 283-290
Zijlstra C, Donkers-Venne DTHM and Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized ampli ed region (SCAR) based PCR assays. Nematology 2: 847-853
Zijlstra C, Uenk BJ and van Silfhout CH (1995) A reliable, precise method to di. erentiate species of root-knot nema-todes in mixtures on the basis of ITS-RFLPs. Fundamental and Applied Nematology 20: 59-63
Zijlstra C, Lever AEM, Uenk BJ and Van Silfhout CH (1997) Di. erences between ITS regions of isolates of root-knot nematodes Meloidogyne hapla and M. chitwoodi. Phytopathology 85: 1231-1237
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hübschen, J., Kling, L., Ipach, U. et al. Development and validation of species-specific primers that provide a molecular diagnostic for virus-vector longidorid nematodes and related species in German viticulture. European Journal of Plant Pathology 110, 883–891 (2004). https://doi.org/10.1007/s10658-004-4841-x
Issue Date:
DOI: https://doi.org/10.1007/s10658-004-4841-x