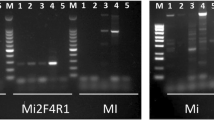
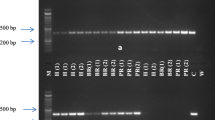
Abstract
The stubby root nematode, Paratrichodorus allius, is an important plant-parasitic nematode that feeds on plant roots and transmits Tobacco rattle virus to potato. Identification of P. allius based on morphometric measurements requires taxonomic knowledge, while previously developed molecular techniques require a well-equipped laboratory. A new recombinase polymerase amplification (RPA) assay was developed in this study, which requires minimal sample preparation, low temperature (37–42 °C), and short time duration (20–40 min) for detection of P. allius. RPA primers were designed targeting the internal transcribed spacer (ITS) region, and conditions were optimized to amplify DNA at 40 °C in 20 min. RPA products were visualized using agarose gel electrophoresis and SYBR Green I dye. In-silico analysis was conducted to predict the primer specificity. The RPA assay was specific to P. allius only. Detection sensitivity of the agarose gel electrophoresis-based RPA assay was 1/4th portion of a single nematode, and a single nematode by the SYBR Green I-based assay. This assay was validated with P. allius infested potato fields. The developed RPA assay can serve as an efficient tool for rapidly detecting P. allius from infested potato fields to help growers with their management decisions.




Similar content being viewed by others
References
Abd El Wahed, A., A. El-Deeb, M. El-Tholoth, H. Abd El Kader, A. Ahmed, S. Hassan, B. Hoffmann, B. Haas, M.A. Shalaby, F.T. Hufert, and M. Weidmann. 2013. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS ONE 8: e71642. https://doi.org/10.1371/journal.pone.0071642.
Bogale, M., A. Baniya, and P. DiGennaro. 2020. Nematode identification techniques and recent advances. Plants 9: 1260. https://doi.org/10.3390/plants9101260.
Boutsika, K., M.S. Phillips, S.A. MacFarlane, D.J.F. Brown, R.C. Holeva, and V.C. Blok. 2004. Molecular diagnostics of some trichodorid nematodes and associated Tobacco rattle virus. Plant Pathology 53: 110–116. https://doi.org/10.1046/j.1365-3059.2003.00938.x.
Boyle, D.S., D.A. Lehman, L. Lillis, D. Peterson, M. Singhal, N. Armes, M. Parker, O. Piepenburg, and J. Overbaugh. 2013. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. Mbio 4: e00135-e213. https://doi.org/10.1128/mBio.00135-13.
Cabada, M.M., J.L. Malaga, A. Castellanos-Gonzalez, K.A. Bagwell, P.A. Naeger, H.K. Rogers, S. Maharsi, M. Mbaka, and A.C. White Jr. 2017. Recombinase polymerase amplification compared to real-time polymerase chain reaction test for the detection of Fasciola hepatica in human stool. The American Journal of Tropical Medicine and Hygiene 96: 341–346. https://doi.org/10.4269/ajtmh.16-0601.
Cha, D., D. Kim, W. Choi, S. Park, and H. Han. 2020. Point-of-care diagnostic (POCD) method for detecting Bursaphelenchus xylophilus in pinewood using recombinase polymerase amplification (RPA) with the portable optical isothermal device (POID). PLoS ONE 15: e0227476. https://doi.org/10.1371/journal.pone.0227476.
Charlton, B. A., Ingham, R. E., David, N. L., Wade, N. M., and McKinley, N. 2010. Effects of in-furrow and water-run oxamyl on Paratrichodorus allius and corky ringspot disease of potato in the Klamath basin. Journal of Nematology 42:1–7.
Chi, Y.K., W. Zhao, M.D. Ye, F. Ali, T. Wang, and R.D. Qi. 2020. Evaluation of recombinase polymerase amplification assay for detecting Meloidogyne javanica. Plant Disease 104: 801–807. https://doi.org/10.1094/PDIS-07-19-1473-RE.
De Ley, I.T., G. Karssen, P. De Ley, A. Vierstraete, L. Waeyenberge, M. Moens, and J. Vanfleteren. 1999. Phylogenetic analyses of internal transcribed spacer region sequences within Meloidogyne. Journal of Nematology 3: 530–531.
Decraemer, W., and P. Baujard. 1998. A polytomous key for the identification of species of the family Trichodoridae Thorne, 1935 (Nematoda: Triplonchida). Fundamental and Applied Nematology 21: 37–62.
Decraemer, W., and R.T. Robbins. 2007. The who, what and where of Longidoridae and Trichodoridae. Journal of Nematology 39: 295–297.
Decreamer, W. 1995. The family Trichodoridae: Stubby-root and virus vector nematodes, 360. Boston: Kluwer Academic Publishers.
Duarte, I.M., M.T.M. Almeida, M.M. Duarte, D.J.F. Brown, and R. Neilson. 2011. Molecular diagnosis of trichodorid species from Portugal. Plant Pathology 60: 586–594. https://doi.org/10.1111/j.1365-3059.2010.02401.x.
Elkins, K.M. 2011. An in silico DNA cloning experiment for the biochemistry laboratory. Biochemistry and Molecular Biology Education 39: 211–215.
Ensen, H.J., and T.C. Allen Jr. 1964. Transmission of tobacco rattle virus by a stubby-root nematode, Trichodorus allius. Plant Disease Reporter 48: 333–334.
FAO. 2022. World food and agriculture - statistical yearbook. Rome: Food and Agriculture Organization of the United Nations (FAO).
Glais, L., and E. Jacquot. 2015. Detection and characterization of viral species/subspecies using isothermal recombinase polymerase amplification (RPA) assays. Plant Pathology: Techniques and Protocols 1302: 207–225. https://doi.org/10.1007/978-1-4939-2620-6_16.
Goraya, M., Yan, G., Plaisance, A., and Handoo, Z. 2023. Identification and reproduction of dagger nematode, Xiphinema americanum, in potato. Nematology (published online ahead of print 2023). https://doi.org/10.1163/15685411-bja10281
Hall, T., I. Biosciences, and C.J.G.B.B. Carlsbad. 2011. BioEdit: An important software for molecular biology. Global Ecology and Research Foundation Bulletin of Biosciences 2: 60–61.
Harris, L.D., and J.D. Griffith. 1989. UvsY protein of bacteriophage T4 is an accessory protein for in vitro catalysis of strand exchange. Journal of Molecular Biology 206: 19–27. https://doi.org/10.1016/0022-2836(89)90520-2.
Holeva, R., M.S. Phillips, R. Neilson, D.J.F. Brown, V. Young, K. Boutsika, and V.C. Blok. 2006. Real-time PCR detection and quantification of vector trichodorid nematodes and Tobacco rattle virus. Molecular and Cellular Probes 20: 203–211. https://doi.org/10.1016/j.mcp.2005.12.004.
Huang, D., G. Yan, and A.M. Skantar. 2017a. a. Development of real-time and conventional PCR assays for identifying stubby root nematode Paratrichodorus allius. Plant Disease 101: 964–972. https://doi.org/10.1094/PDIS-10-16-1431-RE.
Huang, D., G. Yan, N. Gudmestad, and A. Skantar. 2017b. b. Quantification of Paratrichodorus allius in DNA extracted from soil using TaqMan Probe and SYBR Green real-time PCR assays. Nematology 19: 987–1001. https://doi.org/10.1163/15685411-00003101.
Huang, D., G. Yan, N. Gudmestad, J. Whitworth, K. Frost, C. Brown, W. Ye, P. Agudelo, and W. Crow. 2018. Molecular characterization and identification of stubby root nematode species from multiple states in the United States. Plant Disease 102: 2101–2111. https://doi.org/10.1094/PDIS-10-17-1668-RE.
Huang, D., G. Yan, N. Gudmestad, W. Ye, J. Whitworth, K. Frost, W. Crow, and A. Hajihassani. 2019. Developing a one-step multiplex PCR assay for rapid detection of four stubby-root nematode species, Paratrichodorus allius, P. minor, P. porosus, and Trichodorus obtusus. Plant Disease 103: 404–410.
Ingham, R.E., P.B. Hamm, R.E. Williams, and W.H. Swanson. 2000. Control of Paratrichodorus allius and corky ringspot disease of potato in the Columbia basin of Oregon. Journal of Nematology 32: 566–575.
Jenkins, W.R. 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter 48: 692.
Jensen, H. J., Koepsell, P. A., and Allen, T. C. 1974. Tobacco rattle virus and nematode vectors in Oregon. Plant Disease Reporter 58: 269–271.
Ju, Y., Y. Lin, G. Yang, H. Wu, and Y. Pan. 2019. Development of recombinase polymerase amplification assay for rapid detection of Meloidogyne incognita, M. javanica, M. arenaria, and M. enterolobii. European Journal of Plant Pathology 155: 1155–1163. https://doi.org/10.1007/s10658-019-01844-6.
Kibbe, W.A. 2007. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Research 35: W43–W46. https://doi.org/10.1093/nar/gkm234.
Kirk, W.W., S.L. Gieck, J.M. Crosslin, and P.B. Hamm. 2008. First report of corky ringspot caused by tobacco rattle virus on potatoes (Solanum tuberosum) in Michigan. Plant Disease 92: 485. https://doi.org/10.1094/PDIS-92-3-0485B.
Kumari, S., and S.A. Subbotin. 2012. Molecular characterization and diagnostics of stubby root and virus vector nematodes of the family Trichodoridae (Nematoda: Triplonchida) using ribosomal RNA genes. Plant Pathology 61: 1021–1031. https://doi.org/10.1111/j.1365-3059.2012.02598.x.
Lawaju, B., Yan, G., and Whitworth, J. 2023. Development of a droplet digital PCR assay for detection and quantification of stubby root nematode, Paratrichodorus allius in soil. Plant Disease. https://doi.org/10.1094/PDIS-03-23-0439-SR
Lima, F. S. O., Mattos, V. S., Silva, E. S., Carvalho, M. A. S., Teixeira, R. A., Silva, J. C., and Correa, V. R. 2018. Nematodes affecting potato and sustainable practices for their management. Potato: From Incas to All Over the World 107. https://doi.org/10.5772/intechopen.73056
Liu, Y., T. Lei, Z. Liu, Y. Kuang, J. Lyu, and Q. Wang. 2016. A novel technique to detect EGFR mutations in lung cancer. International Journal of Molecular Science 17: 792. https://doi.org/10.3390/ijms17050792.
Liu, D., H. Shen, Y. Zhang, D. Shen, M. Zhu, Y. Song, Z. Zhu, and C. Yang. 2021. A microfluidic-integrated lateral flow recombinase polymerase amplification (MI-IF-RPA) assay for rapid COVID-19 detection. Lab on a Chip 21: 2019–2026. https://doi.org/10.1039/D0LC01222J.
Lobato, I.M., and C.K. O’Sullivan. 2018. Recombinase polymerase amplification: Basics, applications and recent advances. Trac Trends in Analytical Chemistry 98: 19–35. https://doi.org/10.1016/j.trac.2017.10.015.
Lopez-Nicora, H.D., T. Mekete, N. Sekora, and T.L. Niblack. 2014. First report of the stubby-root nematode (Paratrichodorus allius) from a corn field in Ohio. Plant Disease 98: 1164–1164. https://doi.org/10.1094/PDIS-11-13-1180-PDN.
Mayboroda, O., A.G. Benito, J.S. del Rio, M. Svobodova, S. Julich, H. Tomaso, C.K. Sullivan, and I. Katakis. 2016. Isothermal solid-phase amplification system for detection of Yersinia pestis. Analytical and Bioanalytical Chemistry 408: 671–676. https://doi.org/10.1007/s00216-015-9177-1.
Mojtahedi, H., and G.S. Santo. 1999. Ecology of Paratrichodorus allius and its relationship to the corky ring-spot disease of potato in the Pacific Northwest. American Journal of Potato Research 76: 273–280. https://doi.org/10.1007/BF02853625.
Mojtahedi, H., J.M. Crosslin, G.S. Santo, C.R. Brown, and P.E. Thomas. 2001. Pathogenicity of Washington and Oregon isolates of tobacco rattle virus on potato. American Journal of Potato Research 78: 183–190. https://doi.org/10.1007/BF02883543.
Mojtahedi, H., R.A. Boydston, P.E. Thomas, J.M. Crosslin, G.S. Santo, E. Riga, and T.L. Anderson. 2003. Weed hosts of Paratrichodorus allius and tobacco rattle virus in the Pacific Northwest. American Journal of Potato Research 80: 379–385. https://doi.org/10.1007/BF02854249.
Mondal, D., P. Ghosh, M.A.A. Khan, F. Hossain, S. Böhlken-Fascher, G. Matlashewski, A. Kroeger, P. Olliaro, and A. Abd El Wahed. 2016. Mobile suitcase laboratory for rapid detection of Leishmania donovani using recombinase polymerase amplification assay. Parasites & Vectors 9: 1–8. https://doi.org/10.1186/s13071-016-1572-8.
Moyo, L., G. Raikhy, A. Hamid, I. Mallik, N.C. Gudmestad, S. Gray, and H.R. Pappu. 2022. Phylogenetics of tobacco rattle virus isolates from potato (Solanum tuberosum L.) in the USA: A multi-gene approach to evolutionary lineage. Virus Genes 58: 42–52. https://doi.org/10.1007/s11262-021-01875-4.
Oliveira, C.M.G., A.R. Monteiro, and V.C. Blok. 2011. Morphological and molecular diagnostics for plant-parasitic nematodes: Working together to get the identification done. Tropical Plant Pathology 36: 65–73. https://doi.org/10.1590/S1982-56762011000200001.
Perez, E.E., D.P. Weingartner, and R. McSorley. 2000. Correlation between Paratrichodorus minor population levels and corky ringspot symptoms on potato. Nematropica 30: 247–254.
Piepenburg, O., C.H. Williams, D.L. Stemple, and N.A. Armes. 2006. DNA detection using recombination proteins. PLoS Biology 4: 1099–1100. https://doi.org/10.1371/journal.pbio.0040222.
Plaisance, A., and Yan, G. P. 2015. Comparison of two nematode extraction techniques. In Abstracts of the 54th annual meeting of the Society of Nematologists, East Lansing, MI, USA 120.
Ploeg, A.T., and W. Decraemer. 1997. The occurrence and distribution of trichodorid nematodes and their associated tobraviruses in Europe and the former Soviet Union. Nematologica 43: 228–251.
Riga, E., E. Karanastasi, C. Marcelo, G. Oliveira, and R. Neilson. 2007. Molecular identification of two stubby root nematode species. American Journal of Potato Research 84: 161–167. https://doi.org/10.1007/BF02987139.
Singpanomchai, N., Y. Akeda, K. Tomono, A. Tamaru, P. Santanirand, and P. Ratthawongjirakul. 2019. Naked eye detection of the Mycobacterium tuberculosis complex by recombinase polymerase amplification—SYBR green I assays. Journal of Clinical Laboratory Analysis 33: 1–8. https://doi.org/10.1002/jcla.22655.
Subbotin, S.A. 2019. Recombinase polymerase amplification assay for rapid detection of the root-knot nematode Meloidogyne enterolobii. Nematology 21: 243–251.
Subbotin, S.A., and J. Burbridge. 2021. Sensitive, accurate and rapid detection of the northern root-knot nematode, Meloidogyne hapla, using recombinase polymerase amplification assays. Plants 10: 1–12. https://doi.org/10.3390/plants10020336.
Subbotin, S.A., L. Waeyenberge, and M. Moens. 2013. Molecular systematics. Plant. Nematology 2: 41–58.
Wang, T.Y., L. Wang, J.H. Zhang, and W.H. Dong. 2011. A simplified universal genomic DNA extraction protocol suitable for PCR. Genetics and Molecular Research 10: 519–525.
Yamanaka, E.S., L.A. Tortajada-Genaro, and Á. Maquieira. 2017. Low-cost genotyping method based on allele-specific recombinase polymerase amplification and colorimetric microarray detection. Microchimica Acta 184: 1453–1462. https://doi.org/10.1007/s00604-017-2144-0.
Yan, G., and R.W. Smiley. 2010. Distinguishing Heterodera filipjevi and H. avenae using polymerase chain reaction-restriction fragment length polymorphism and cyst morphology. Phytopathology 100: 216–224. https://doi.org/10.1094/PHYTO-100-3-0216.
Yan, G., R.W. Smiley, P.A. Okubara, A.M. Skantar, and C.L. Reardon. 2013. Developing a real-time PCR assay for detection and quantification of Pratylenchus neglectus in soil. Plant Disease 97: 757–764.
Yan, L., J. Zhou, Y. Zheng, A.S. Gamson, B.T. Roembke, S. Nakayama, and H.O. Sintim. 2014. Isothermal amplified detection of DNA and RNA. Molecular BioSystems 10: 970–1003.
Yan, G., A. Plaisance, D. Huang, A. Upadhaya, N.C. Gudmestad, and Z.A. Handoo. 2016. First report of the stubby root nematode Paratrichodorus allius on potato in North Dakota. Plant Disease 100: 1247. https://doi.org/10.1094/PDIS-11-15-1350-PDN.
Zhang, S., A. Sun, B. Wan, Y. Du, Y. Wu, A. Zhang, D. Jiang, P. Ji, Z. Wei, G. Zhuang, and G. Zhang. 2020. Development of a directly visualized recombinase polymerase amplification–SYBR Green I method for the rapid detection of African swine fever virus. Frontiers in Microbiology 11: 1–9. https://doi.org/10.3389/fmicb.2020.602709.
Zheng, Y., P. Hu, H. Ren, H. Wang, Q. Cao, Q. Zhao, H. Li, H. Zhang, Z. Liu, Y. Li, and C. Wang. 2021. RPA-SYBR Green I based instrument-free visual detection for pathogenic Yersinia enterocolitica in meat. Analytical Biochemistry 621: 1–6. https://doi.org/10.1016/j.ab.2021.114157.
Acknowledgements
The authors thank Dr. Intiaz Chowdhury and Dr. Bisho Lawaju for valuable suggestions in this research. The authors are grateful to Addison Plaisance for collecting the soil samples from North Dakota. We are also thankful to Richard Quick for his technical assistance in collecting nematodes from Washington. This research was supported by the U.S. Department of Agriculture (USDA)’s Agricultural Marketing Service (AMS) through grant number 19-425. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goraya, M., Yan, G., Whitworth, J. et al. Advancing Nematode Identification on Potato: An Isothermal Recombinase Polymerase Amplification Assay for Stubby Root Nematode, Paratrichodorus allius. Am. J. Potato Res. 101, 52–64 (2024). https://doi.org/10.1007/s12230-023-09940-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-023-09940-4