Abstract
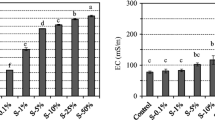
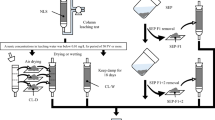
Massive quantities of alkaline rocks are excavated from urban coastal and mountain areas to make underground spaces available for infrastructure projects; however, such excavated rock often releases arsenic. In the present study, arsenic release from the excavated rocks with steel slag was investigated using dialysis and batch leaching tests to understand where arsenic is immobilized and which components in the steel slag suppress arsenic release from the excavated rock. Dialysis test indicated that the addition of steel slag at 10 wt% could suppress arsenic release at a level greater than 66%. The total arsenic content in the steel slag did not increase as compared with that before the test. Sequential extraction analysis indicated that the arsenic released during the dialysis test is mainly derived from arsenic fraction 1 (nonspecifically bound) due to the higher amount of this arsenic fraction in the excavated rock with the steel slag. Moreover, the steel slag extract could suppress arsenic release from the excavated rock and remove the arsenic from aqueous solution. The pH dependence test further indicated that the arsenic immobilized by the steel slag extract was stable under alkaline pH conditions. The levels of arsenic release decreased with increasing calcium release from the steel slag regardless of the type of excavated rock with an alkaline pH and were particularly seen at calcium released > 500 mg kg−1. These results indicate that the arsenic immobilization could be occurred not on the surface of steel slag, but on the excavated rock, and the calcium dissolved from the steel slag regulates the behavior of arsenic release from the surface of excavated rock. The findings of the present study suggest that the steel slag could be utilized to enable the reuse of excavated sedimentary and metamorphic rock of alkaline pH for the control of arsenic release.







Similar content being viewed by others
References
Alexandratos, V. G., Elzinga, E. J., & Reeder, R. J. (2007). Arsenate uptake by calcite: microscopic and spectroscopic characterization of adsorption and incorporation mechanisms. Geochimica et Cosmochimica Acta, 71, 4172–4187.
Bothe, J. V., Jr., & Brown, P. W. (1999). Arsenic immobilization by calcium arsenate formation. Environ Sci Technol, 33, 3806–3811.
Cai, Y., Zhang, H., Yuan, G., & Li, F. (2017). Sources, speciation and transformation of arsenic in the gold mining impacted Jiehe River, China. Appl Geochem, 84, 254–261.
Cheng, H., Hu, Y., Luo, J., Xu, B., & Zhao, J. (2009). Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems (Review). J Hazard Mater, 165, 13–26.
Fendorf, S., Eick, M. J., Grossl, P., & Sparks, D. L. (1997). Arsenate and chromate retention mechanisms on goethite. 1 Surface structure. Environ Sci Technol, 31, 315–320.
Hartley, W., Dickinson, N. M., Riby, P., & Lepp, N. W. (2009). Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut, 157, 2654–2662.
Kamata, A., & Katoh, M. (2019). Arsenic release from marine sedimentary rock after excavation from urbanized coastal areas: oxidation of framboidal pyrite and subsequent natural suppression of arsenic release. Sci Total Environ, 670, 752–759.
Katoh, M., Matsuoka, H., & Sato, T. (2015a). Stability of lead immobilized by apatite in lead-containing rhizosphere soil of buckwheat (Fagopyrum esculentum) and hairy vetch (Vicia villosa). Int J Phytoremediat, 17, 604–611.
Katoh, M., Wang, Y., Kitahara, W., & Sato, T. (2015b). Impact of phosphorus and water-soluble organic carbon in cattle and swine manure composts on lead immobilization in soil. Environ Technol, 36, 1943–1953.
Katoh, M., Tsuda, K., Matsumoto, N., & Sato, T. (2016). Formation of pyromorphite and lead mobilization in contaminated soils amended with hydroxyapatite in the presence of iron oxyhydroxide and water percolation. Water Air Soil Pollut, 227, 470.
Katoh, M., Moriguchi, S., Takagi, N., Akashi, Y., & Sato, T. (2018). Simultaneous control of cadmium release and acidic pH neutralization in excavated sedimentary rock with concurrent oxidation of pyrite using steel slag. J Soils Sedim, 18, 1194–1204.
Katsumi, T. (2015). Soil excavation and reclamation in civil engineering: environmental aspects. Soil Sci Plant Nutr, 61, 22–29.
Kumpiene, J., Montesinos, I. C., Lagerkvist, A., & Maurice, C. (2007). Evaluation of the critical factors controlling stability of chromium, copper, arsenic and zinc in iron-treated soil. Chemosphere, 67, 410–417.
Lockwood, C. L., Mortimer, R. J. G., Stewart, D. I., Mayes, W. M., Peacock, C. L., Polya, D. A., et al. (2014). Mobilisation of arsenic from bauxite residue (red mud) affected soils: effect of pH and redox conditions. Appl Geochem, 51, 268–277.
Nickson, R. T., Mcarthur, J. M., Ravenscroft, P., Burgess, W. G., & Ahmed, K. M. (2000). Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem, 15, 403–413.
Ogawa, S., Kaoth, M., Numako, C., Kitahara, K., Miyazaki, S., & Sato, T. (2016). Immobilization of antimony(III) in oxic soil using combined application of hydroxyapatite and ferrihydrite. Water Air Soil Pollut, 227, 124.
Ogawa, S., Sato, T., & Katoh, M. (2018). Formation of a lead-insoluble phase, pyromorphite, by hydroxyapatite during lead migration through the water-unsaturated soils of different lead mobilities. Environ Sci Pollut Res, 25, 7662–7671.
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Techniques and Methods, book 6, chap. A43, pp 497.
Piatak, N. M., Parsons, M. B., & Seal, R. R., II. (2015). Characteristics and environmental aspects of slag: a review. Appl istry, 57, 236–266.
Sharma, P., & Kappler, A. (2011). Desorption of arsenic from clay and humic acid-coated clay by dissolved phosphate and silicate. J Contam Hydrol, 126, 216–225.
Shimizu, S., Sato, T., & Katoh, M. (2018). Formation of a lead insoluble phase using an immobilization material and its maximization in soil under unsaturated moisture conditions. J Soils Sedim, 18, 1052–1059.
Song, S., Lopez-Valdivieso, A., Hernandez-Campos, D. J., Peng, C., Monroy-Fernandez, M. G., & Razo-Soto, I. (2006). Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite. Water Res, 40, 364–372.
Streat, M., Hellgardt, K., & Newton, N. L. R. (2008). Hydrous ferric oxide as an adsorbent in water treatment: Part 3: Batch and mini-column adsorption of arsenic, phosphorus, fluorine and cadmium ions. Process Saf Environ, 86, 21–30.
Suzuki, S., & Katoh, M. (2020). Estimation of potential arsenic leaching from its phases in excavated sedimentary and metamorphic rocks. Environ Geochem Health, 42, 407–418.
Tabelin, C. B., Hashimoto, A., Igarashi, T., & Yoneda, T. (2014). Leaching of boron, arsenic and selenium from sedimentary rocks: I. effects of contact time, mixing speed and liquid-to-solid ratio. Sci Total Environ, 472, 620–629.
Tabelin, C. B., Igarashi, T., Villacorte-Tabelin, M., Park, I., Opiso, E. M., Ito, M., et al. (2018). Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: a review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci Total Environ, 645, 1522–1553.
Tangviroon, P., Hayashi, R., & Igarashi, T. (2017). Effects of additional layer(s) on the mobility of arsenic from hydrothermally altered rock in laboratory column experiments. Water Air Soil Pollut, 228, 191.
Wenzel, W. W., Kirchbaumer, N., Prohaska, T., Stingeder, G., Lombi, E., & Adriano, D. C. (2001). Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta, 436, 309–323.
Xenidis, A., Stouraiti, C., & Papassiopi, N. (2010). Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J Hazard Mater, 177, 929–937.
Acknowledgments
The authors are grateful to Mr. T. Miura for rock sample collection and acknowledge to Prof. F. Li and Prof. T. Yamada (Gifu University) for allowing the use of the ICP-MS. The ICP-OES was made available by the Division of Instrumental Analysis at Gifu University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hada, S., Moriguchi, S., Akashi, Y. et al. Suppression of arsenic release from alkaline excavated rock by calcium dissolved from steel slag. Environ Geochem Health 42, 3983–3993 (2020). https://doi.org/10.1007/s10653-020-00657-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00657-5