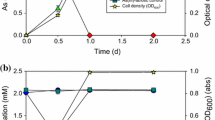
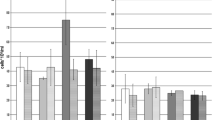
Abstract
Hot Springs have unique geochemical features. Microorganisms-mediated arsenite oxidation is one of the major biogeochemical processes occurred in some hot springs. This study aimed to understand the diversities of genes and microorganisms involved in arsenite oxidation from the outlet of an untraversed hot spring located at an altitude of 4226 m. Microcosm assay indicated that the microbial community from the hot spring was able to efficiently oxidize As(III) using glucose, lactic acid, yeast extract or sodium bicarbonate as the sole carbon source. The microbial community contained 7 phyla of microorganisms, of which Proteobacteria and Firmicutes are largely dominant; this composition is unique and differs significantly from those of other described hot springs. Twenty one novel arsenite oxidase genes were identified from the samples, which are affiliated with the arsenite oxidase families of α-Proteobacteria, β-Proteobacteria or Archaea; this highlights the high diversity of the arsenite-oxidizing microorganisms from the hot spring. A cultivable arsenite-oxidizer Chelatococcu sp. GHS311 was also isolated from the sample using enrichment technique. It can completely convert 75.0 mg/L As(III) into As(V) in 18 days at 45 °C. The arsenite oxidase of GHS311 shares the maximal sequence identity (84.7%) to that of Hydrogenophaga sp. CL3, a non-thermotolerant bacterium. At the temperature lower than 30 °C or higher than 65 °C, the growth of this strain was completely inhibited. These data help us to better understand the diversity and functional features of the thermophilic arsenite-oxidizing microorganisms from hot springs.





Similar content being viewed by others
References
Anderson GL, Williams J, Hille R (1992) The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J Biol Chem 267:23674–23682
Ao L, Zeng XC, Nie Y, Mu Y, Zhou L, Luo X (2014) Flavobacterium arsenatis sp. nov., a novel arsenic-resistant bacterium from high-arsenic sediment. Int J Syst Evol Microbiol 64:3369–3374
Baek SH, Kim KH, Yin CR, Jeon CO, Im WT, Kim KK (2003) Isolation and characterization of bacteria capable of degrading phenol and reducing nitrate under low-oxygen conditions. Curr Microbiol 47(6):462–466
Bahar MM, Megharaj M, Naidu R (2012) 1Arsenic bioremediation potential of a new arsenite-oxidizing bacterium Stenotrophomonas sp. MM-7 isolated from soil. Biodegradation 23:803–812
Barra CA, Topp E, Grenni P (2015) Pharmaceuticals in the environment: biodegradation and effects on natural microbial communities. A review. J Pharm Biomed 106:25–36
Beam JP, Jay ZJ, Schmid MC, Rusch DB, Romine MF, Jennings Rde R et al. (2016) Ecophysiology of an uncultivated lineage of aigarchaeota from an oxic, hot spring filamentous ‘streamer’community. ISME J 10(1):210–224
Brock TD (1978) Thermophilic microorganisms and life at high temperatures. Springer 230(4722):132–138
Brock TD, Freeze H (1969) Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol 98(1):289–297
Bundschuh J, Maity JP (2015) Geothermal arsenic: occurrence, mobility and environmental implications. Renew Sust Energ Rev 42:1214–1222
Chang JS (2015) Biotransformation of arsenite and bacterial aox activity in drinking water produced from surface water of floating houses: arsenic contamination in Cambodia. Environ Pollut 206:315–323
Chang JS, Yoon IH, Lee JH, Kim KR, An J, Kim KW (2010) Arsenic detoxification potential of aox genes in arsenite-oxidizing bacteria isolated from natural and constructed wetlands in the Republic of Korea. Environ Geochem Health 32:95–105
Chen X, Zeng XC, Wang J, Deng Y, Ma T, Guoji E et al. (2017) Microbial communities involved in arsenic mobilization and release from the deep sediments into groundwater in Jianghan plain, Central China. Sci Total Environ 579:989–999
Cole JK, Peacock JP, Dodsworth JA, Williams AJ, Thompson DB, Dong H (2013) Sediment microbial communities in great boiling spring are controlled by temperature and distinct from water communities. ISME J 7(4):718–729
Connon SA, Koski AK, Neal AL, Wood SA, Magnuson TS (2008) Ecophysiology and geochemistry of microbial arsenic oxidation within a high arsenic, circumneutral hot spring system of the Alvord desert. FEMS Microbial Ecol 64(1):117–128
Costa PS, Reis MP, Ávila MP, Leite LR, de Araújo FM, Salim AC et al. (2015) Metagenome of a microbial community inhabiting a metal-rich tropical stream sediment. PLos One 10(3):236–240
D’Arcy R, Amend JP (2014) Geochemistry and microbial ecology in alkaline hot springs of Ambitle Island, Papua New Guinea. Extremophiles 18(4):763–778
Donahoe-Christiansen J, D’Imperio S, Jackson CR, Inskeep WP, McDermott TR (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Ellis PJ, Conrads T, Hille R, Kuhn P (2001) Crystal structure of the 100 kDa arsenite oxidase from Alcaligenes faecalis in two crystal forms at 1.64 Å and 2.03 Å. Structure 9:125–132
Engel AS, Johnson LR, Porter ML (2013) Arsenite oxidase gene diversity among Chloroflexi and Proteobacteria from El Tatio Geyser Field, Chile. FEMS Microbiol Ecol 83:745–756
Essington ME (2015) Soil and water chemistry: an integrative approach. J Nurs Qual Assur 1(1):8–16
Gaisin VA, Grouzdev DS, Namsaraev ZB, Sukhacheva MV, Gorlenko VM, Kuznetsov BB (2016) Biogeography of thermophilic phototrophic bacteria belonging to roseiflexus genus. FEMS Microbiol Ecol 92(3):1538–1547
Garcia-Dominguez E, Mumford A, Rhine ED, Paschal A, Young LY (2008) Novel autotrophic arsenite-oxidizing bacteria isolated from soil and sediments. FEMS Microbiol Ecol 66:401–410
Ghosh D, Bhadury P, Routh J (2015) Diversity of arsenite oxidizing bacterial communities in arsenic-rich deltaic aquifers in West Bengal, India. Front Microbiol 5(5):1–14.
Gihring TM, Banfield JF (2001) Arsenite oxidation and arsenate respiration by a new thermus isolate. FEMS Microbiol Lett 204(2):335–340
Gihring TM, Druschel GK, Mccleskey RB, Hamers RJ, Banfield JF (2001) Rapid arsenite oxidation by Thermus aquaticus and Thermus thermophilus: field and laboratory investigations. Environ Sci Technol 35(19):3857–3862
Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresour Technol 89(1):17–34
Hall JR, Mitchell KR, Jackson-Weaver O, Kooser AS, Cron BR, Crossey LJ et al. (2008) Molecular characterization of the diversity and distribution of a thermal spring microbial community by using rRNA and metabolic genes. Appl Environ Microbiol 74(15):4910–4922
Halter D, Cordi A, Gribaldo S, Gallien S, Goulhenchollet F, Heinrichsalmeron A et al. (2011) Taxonomic and functional prokaryote diversity in mildly arsenic-contaminated sediments. Res Microbiol 162(9):877–887
Hamamura N, Fukushima K, Itai T (2013) Identification of antimony- and arsenic-oxidizing bacteria associated with antimony mine tailing. Microbes Environ 28(2):257–263
Hamamura N, Macur RE, Korf S, Ackerman G, Taylor WP, Kozubal M et al. (2009) Linking microbial oxidation of arsenic with detection and phylogenetic analysis of arsenite oxidase genes in diverse geothermal environments. Environ Microbiol 11:421–431
Heinrich-Salmeron A, Cordi A, Brochier-Armanet C, Halter D, Pagnout C, Abbaszadeh-Fard E et al. (2011) Unsuspected diversity of arsenite-oxidizing bacteria revealed by a widespread distribution of the aoxB gene in prokaryotes. Appl Environ Microbiol 77(13):4685–4692
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP et al. (2013) A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One 8(1):e53350
Inskeep WP, Jay ZJ, Macur RE, Clingenpeel S, Tenney A, Lovalvo D et al. (2015) Geomicrobiology of sublacustrine thermal vents in Yellowstone Lake: geochemical controls on microbial community structure and function. Front Microbial 6:1044
Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM (2007) Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ Microbiol 9:934–943
Jackson CR, Langner HW, Donahoe-Christiansen J, Inskeep WP, McDermott TR (2001) Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ Microbiol 3:532–542
Jiang D, Li P, Jiang Z, Dai X, Zhang R, Wang Y et al. (2015) Chemolithoautotrophic arsenite oxidation by a thermophilic Anoxybacillus flavithermus strain TCC9-4 from a hot spring in Tengchong of Yunnan, China. Front Microbiol 6:360
Jiang Z, Li P, Van NJD, Zhang P, Zhou J, Wang Y et al. (2016) Microbial communities and arsenic biogeochemistry at the outflow of an alkaline sulfide-rich hot spring. Sci Rep 6:25262
Katrin H, William AM, Matthew BS, Frank K, Simon F, John WM (2014) Microbial contributions to coupled arsenic and sulfur cycling in the acid-sulfide hot spring champagne pool, new zealand. Front Microbiol 5(5):569
Landrum JT, Bennett PC, Engel AS, Alsina MA, Pastén PA, Milliken K (2009) Partitioning geochemistry of arsenic and antimony, El Tatio Geyser Field, Chile. Appl Geochem 24(24):664–676
Langner HW, Jackson CR, McDermott TR, Inskeep WP (2001) Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ Sci Technol 35:3302–3309
LaPara TM, Alleman JE (1999) Thermophilic aerobic biological wastewater treatment. Water Res 33(4):895–908
Lau MC, Aitchison JC, Pointing SB (2009) Bacterial community composition in thermophilic microbial mats from five hot springs in central Tibet. Extremophiles 13(1):139–149
Lawati WMA, Rizoulis A, Eiche E, Boothman C, Polya DA, Lloyd JR et al. (2012) Characterisation of organic matter and microbial communities in contrasting arsenic-rich holocene and arsenic-poor pleistocene aquifers, red river delta, vietnam. Appl Geochem 27(1):315–325
Lefevre E, Bossa N, Wiesner MR, Gunsch CK (2016) A review of the environmental implications of in situ, remediation by nanoscale zero valent iron (nzvi): behavior, transport and impacts on microbial communities. Sci Total Environ 565:889–901
Li Y, Guo H, Hao C (2014) Arsenic release from shallow aquifers of the Hetao basin, Inner Mongolia: evidence from bacterial community in aquifer sediments and groundwater. Ecotoxicology 23(10):1900–1914
Li P, Wang Y, Dai X, Zhang R, Jiang Z, Jiang D et al. (2015) Microbial community in high arsenic shallow groundwater aquifers in Hetao basin of inner Mongolia, China. PLoS One 10:e0125844
Li H, Zeng XC, He Z, Chen X, E G, Han Y et al. (2016) Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res 101:393–401
Marco PD, Pacheco CC, Figueiredo AR, Moradas-Ferreira P (2004) Novel pollutant-resistant methylotrophic bacteria for use in bioremediation. FEMS Microbiol Lett 234(1):75–80
Milton HR (1977) Extreme environments: mechanisms of microbial adaptation. Trans Chin Soc Agric Engng 27(5):373–377
Mu Y, Pan Y, Shi W, Liu L, Jiang Z, Luo X, Li WJ (2016) Luteimonas arsenica sp. nov., a new arsenic-tolerant bacterium isolated from arsenic contaminated soil. Int J Syst Evol Microbiol 66:2291–2296
Oremland RS, Hoeft SE, Santini JM, Bano N, Hollibaugh RA, Hollibaugh JT (2002) Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl Environ Microbiol 68:4795–4802
Paul D, Poddar S, Sar P (2014) Characterization of arsenite-oxidizing bacteria isolated from arsenic-contaminated groundwater of West Bengal. J Environ Sci Health Part A 49:1481–1492
Plugge CM, Zoetendal EG, Stams AJ (2000) Caloramator coolhaasii sp. nov. A glutamate-degrading, moderately thermophilic anaerobe. Int J Syst Evol Microbiol 3:1155–1162
Quéméneur M, Cébron A, Billard P, Battaglia-Brunet F, Garrido F, Leyval C et al. (2010) Population structure and abundance of arsenite-oxidizing bacteria along an arsenic pollution gradient in waters of the upper Isle River Basin, France. Appl Environ Microbiol 76:4566–4570
Quéméneur M, Heinrich-Salmeron A, Muller D, Lièvremont D, Jauzein M, Bertin PN et al. (2008) Diversity surveys and evolutionary relationships of aoxB genes in aerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74(14):4567–4573
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409(6823):1092-1101.
Santini JM, Sly LI, Schnagl RD, Macy JM (2000) A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66:92–97
Simonin M, Richaume A (2015) Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review. Environ Sci Pollut Res 22(18):13710–13723
Singh A, Subudhi E (2016) Structural insights of microbial community of Deulajhari (India) hot spring using 16s-rrna based metagenomic sequencing. Genom Data 7:101–102
Sultana M, Härtig C, Planer-Friedrich B, Seifert J, Schlömann M (2011) Bacterial communities in Bangladesh aquifers differing in aqueous arsenic concentration. Geomicrobiol J 28(3):198–211
Sultana M, Vogler S, Zargar K, Schmidt AC, Saltikov C, Seifert J et al. (2012) New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch Microbiol 194:623–635
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6(1):1
Urbieta MS, González-Toril E, Bazán ÁA, Giaveno MA, Donati E (2015) Comparison of the microbial communities of hot springs waters and the microbial biofilms in the acidic geothermal area of Copahue (Neuquén, Argentina). Extremophiles 19(2):437–450
Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G et al. (2013) Control of temperature on microbial community structure in hot springs of the tibetan plateau. PLos One 8(5):e62901
Wang L, Man KC, Kwan HS, Hwang JS, Chong KW (2015) Microbial diversity in shallow-water hydrothermal sediments of Kueishan island, taiwan as revealed by pyrosequencing. J Basic Microbiol 55(11):1407–1419
Wemheuer B, Taube R, Akyol P, Wemheuer F, Daniel R (2013) Microbial diversity and biochemical potential encoded by thermal spring metagenomes derived from the Kamchatka Peninsula. Archaea 2013:136714
Wiegel J, Ljungdahl LG, Demain AL (1985) The importance of thermophilic bacteria in biotechnology. Crit Rev Biotechnol 3:39–108
Xu L, Zeng XC, Nie Y, Luo X, Zhou E, Zhou L, Li W (2014) Pontibacter diazotrophicus sp. nov., a novel nitrogen-fixing bacterium of the family cytophagaceae. PLos One 9:e92294
Yoon KS, Tsukada N, Sakai Y, Ishii M, Igarashi Y, Nishihara H (2008) Isolation and characterization of a new facultatively autotrophic hydrogen-oxidizing betaproteobacterium, hydrogenophaga sp. AH-24. FEMS Microbiol Lett 278(1):94–100
Zeng XC, Guoji E, Wang J, Wang N, Chen X, Mu Y et al. (2016) Functions and unique diversity of genes and microorganisms involved in arsenite oxidation from the tailings of a realgar mine. Appl Environ Microbiol 82(24):7019–7029
Zhang G, Liu C, Liu H, Jin Z, Han G, Li L (2008) Geochemistry of the Rehai and Ruidian geothermal waters, Yunnan Province, China. Geothermics 37:73–83
Acknowledgements
This work was financially supported by the General Programs (Grants nos. 41072181, 41472219 and 41272257) and the Foundation for Innovative Research Groups from the National Natural Science Foundation of China (Grant no. 41521001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
No specific permissions were required for the collection of samples from this hot spring. Moreover, this location did not involve endangered and protected species and it is not under regulatory body concerned with protection of wildlife.
Additional information
Ye Yang and Yao Mu have contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, Y., Mu, Y., Zeng, XC. et al. Functional genes and thermophilic microorganisms responsible for arsenite oxidation from the shallow sediment of an untraversed hot spring outlet. Ecotoxicology 26, 490–501 (2017). https://doi.org/10.1007/s10646-017-1779-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1779-2