Abstract
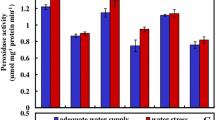
Drought stress is one of the major abiotic stresses that affects plant growth and metabolism adversely around the world. According to this research, the effect of drought stress on the activity of antioxidative enzymes, soluble sugar, protein and lipid peroxidation were studied in leaves of two mangrove plants, Kandelia obovata and Aegiceras corniculatum. The result showed that superoxide dismutase (SOD) and peroxidase (POD) varied significantly between the leaves and roots studied. The activities increased in different stress levels. The production rate of O −·2 changed with the activity of SOD and POD. Lipid peroxidation was enhanced and Glycine betaine (GB) could decrease the level of malonaldehyde in order to reduce the damage of membrane system. The content of soluble sugar and protein also increased under drought stress and GB helped to eliminate the accumulation of them which somehow enhance the ability of defensing the plants under drought stress. These results indicated that antioxidative activity may play an important role in A. corniculatum and K. obovata and that cell membrane in leaves of K. obovata had greater stability than those of A. corniculatum. Exogenous application of GB had positive effects on A. corniculatum and K. obovata under drought stress which could be products exogenously applied to mangrove plants in order to alleviates the adverse effects.






Similar content being viewed by others
References
Allen RD (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol 107(4):1049–1054
Ashihara et al (1997) Compatible solutes and inorganic ions in the mangrove plant avicennia marina and their effects on the activities of enzymes. Zeitschrift für Naturforschung C 52(7–8):433–440
Beaucham C, Fridovic I (1971) Superoxide dismutase—improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276
Bowler C, Montagu MV, Inze D (1992a) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43(1):83–116
Bowler C, Vanmontagu M, Inze D (1992b) Superoxide-dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43(5):83–116
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cha-Um S, Supaibulwattana K, Kirdmanee C (2009) Comparative effects of salt stress and extreme pH stress combined on glycinebetaine accumulation, photosynthetic abilities and growth characters of two rice genotypes. Rice Sci 16(4):274–282
Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5(3):250–257
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34(1):1–20
Chen LZ, Wang WQ (2004) Influence of water logging time on the growth of Kandelia candel seedlings. Acta Oceanol Sinica 23(1):149–158
Cheng H, Wang YS, Ye ZH, Chen DT, Wang YT, Peng YL, Wang LY (2012) Influence of N deficiency and salinity on metal (Pb, Zn and Cu) accumulation and tolerance by Rhizophora stylosa in relation to root anatomy and permeability. Environ Pollut 164:110–117
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30(7):875–885
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gorham J, Bridges J (1995) Effects of calcium on growth and leaf ion concentrations of Gossypium hirsutum grown in saline hydroponic culture. Plant Soil 176(2):219–227
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32(6):481
He KY, Li XC, Huang LB, Zhang YB, Hu XJ (2004) Effects of drought stress on physiological and biochemical indices in five tree species of Magnoliaceae. J Plant Res Environ 13(4):20–23
He BY, Lai TH, Fan HQ, Wang WQ, Zheng HL (2007) Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the Beibu Gulf. Estuar Coast Shelf Sci 74(1–2):254–262
Krauss KW, McKee KL, Lovelock CE, Cahoon DR, Saintilan N, Reef R, Chen L (2014) How mangrove forests adjust to rising sea level. New Phytol 202(1):19–34
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27(4):969–978
Liu RD, Wang YM (2004) Effect of exogenous betaine on physiological index of drought resistance for apricot. J Inner Mong Agric Univ 25(2):69–72
Ma¨kela P, Jokinen K (1998a) Effect of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt- or drought-stressed tomato. Aust J Plant Physiol 25:655–663
Ma¨kela P, Jokinen K (1998b) Foliar application of glycinebetaine—a novel product from sugar beet—as an approach to increase tomato yield. Ind Crops Prod 7:139–148
Minorsky PV (2002) Global warming—effects on plants. Plant Physiol 129(4):1421–1422
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Park EJ, Jeknic Z, Chen THH (2006) Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol 47(6):706–714
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2(6):477–486
Rajashekar CB, Zhou H, Marcum KB, Prakash O (1999) Glycine betaine accumulation and induction of cold tolerance in strawberry (Fragaria X ananassa Duch.) plants. Plant Sci 148(2):175–183
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher-plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Rosenzweig C, Parry ML (1994) Potential impact of climate-change on world food-supply. Nature 367(6459):133–138
Sakamoto A, Murata N (2000) Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51(342):81–88
Siedlecka A, Krupa Z (2002) Functions of enzymes in heavy metal treated plants. Physiol Biochem Met Toxic Toler Plants 303–324
Spalding EP (2010) The inside view on plant growth. Nat Methods 7(7):506–507
Turner NC (1997) Further progress in crop water relations. Adv Agron 58:293–338
Wang Y, Hu S (2013) A new method for fast determination of total soluble sugar content in plant tissue: TBA-TBA-METHOD. J Jinggangshan Univ (Nat Sci) 34(3):37–40
Wang WQ, Xiao Y, Chen LZ, Lin P (2007) Leaf anatomical responses to periodical waterlogging in simulated semidiurnal tides in mangrove Bruguiera gymnorrhiza seedlings. Aquat Bot 86(3):223–228
Wei BX (1999) Alleviation of water stress in beans by exogenous glycine betaine. Plant Sci 148:185–192
Wei JX (2007) Effects of betaine on cold resistance of banana seedlings. Guangdong Agric Sci 7:41–43
Woo S, Yum S, Park HS, Lee TK, Ryu JC (2009) Effects of heavy metals on antioxidants and stress-responsive gene expression in Javanese medaka (Oryzias javanicus). Comp Biochem Physiol Toxicol Pharmacol : CBP 149(3):289–299
Xing WB, Rajashekar CB (1999) Alleviation of water stress in beans by exogenous glycine betaine. Plant Sci 148(2):185–192
Zhan JYY, He LF (2006) Programmed cell death of plant in adversity conditions. Guangxi Agric Sci 37:13–16
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67(1):44–50
Acknowledgments
This research was supported by the Guangzhou Science and Technology Projects (No. 15020024), the National Natural Science Foundation of China (No. 41430966, No. 41076070 and No. 41176101), the key projects in the National Science & Technology Pillar Program in the Eleventh Five-year Plan Period (No. 2012BAC07B0402) and the Knowledge Innovation Programs of the Chinese Academy of Sciences (No. KSCX2-SW-132).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, GF., Wang, YS., Cheng, H. et al. Physiological and biochemical response to drought stress in the leaves of Aegiceras corniculatum and Kandelia obovata . Ecotoxicology 24, 1668–1676 (2015). https://doi.org/10.1007/s10646-015-1470-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1470-4