Abstract
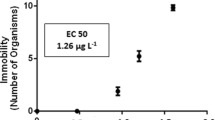
Chinese toad, Bufo bufo gargarizans, is frequently found in rice fields, muddy ponds, wetlands and other aquatic ecosystems in China. Because of its habitat, it has many chances of being exposed to pesticides, such as acetochlor, butachlor, chlorimuron-ethyl, and paraquat, which are extensively used in rice or cereal fields. Amphibians may serve as model organisms for determining the genotoxic effects of pollutants contaminating these areas. In the present study DNA damage was evaluated in the Chinese toad using the comet assay, as a potential tool for the assessment of ecogenotoxicity. The first step was to determine the acute toxicity of the above-mentioned herbicides. In acute tests, tadpoles were exposed to a series of relatively high concentrations of acetochlor, butachlor, chlorimuron-ethyl, and paraquat for 96 h. The LC50 (96 h) of acetochlor, butachlor, chlorimuron-ethyl and paraquat were measured as 0.76, 1.32, 20.1 and 164 mg·l−1, respectively. Also, negative effects on the behavior of tadpoles were observed with acetochlor, butachlor, and paraquat. Secondly, the comet assay was used for detecting DNA damage in Chinese toad tadpoles exposed to sublethal concentrations of four herbicides. Significant (P < 0.05) concentration-dependent increase in DNA damage (as indicated by tail length, tail moment, olive tail moment) were observed from erythrocytes of tadpoles exposed to sublethal concentrations of acetochlor, butachlor, paraquat, and methyl methanesulfonate, except chlorimuron-ethyl. To our knowledge, this is the first report describing the use of Bufo bufo gargarizans for genotoxicity assessment of herbicides.

Similar content being viewed by others
References
Aguilar C, Ferrer I, Borrull F, Marcé RM, Barceló D (1999) Monitoring of pesticides in river water based on samples previously stored in polymeric cartridges followed by on-line solid-phase extraction-liquid chromatography-diode array detection and confirmation by atmospheric pressure chemical ionization mass spectrometry. Anal Chim Acta 386:237–248
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterase and glutathione S-transferase activity in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16:533–539
Bajpayee M, Pandey AK, Parmar D, Mathur N, Seth PK, Dhawan A (2005) Comet assay responses in human lymphocytes are not influenced by the menstrual cycle: a case study in healthy Indian females. Mutat Res 565(2):163–172
Buschini A, Carboni P, Martino A, Poli P, Rossi C (2003) Effects of temperature on baseline and genotoxicant-induced DNA damage in haemocytes of Dreissena polymorpha. Mutat Res 537:81–92
Bushra A, Abul Farah M, Waseem A (2005) Detection of DNA damage by alkaline single cell gel electrophoresis in 2,4-dichlorophenoxyacetic-acid and butachlor-exposed erythrocytes of Clarias batrachus. Ecotoxicol Environ Saf 62:348–354
Capel PD, Ma L, Schroyer BR, Larson SJ, Gilchrist TA (1995) Analysis and detection of the new corn herbicide acetochlor in river water and rain. Environ Sci Technol 29:1702–1705
Cheek AO, Ide CF, Bollinger JE, Rider CV, McLachlan JA (1999) Alteration of leopard frog (Rana pipiens) metamorphosis by the herbicide acetochlor. Arch Environ Contam Toxicol 37:70–77
Colombo A, Orsi F, Bonfanti P (2005) Exposure to the organophosphorus pesticide chlorpyrifos inhibit acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemophere 61:1665–1671
Cotelle S, Ferard JF (1999) Comet assay in genetic ecotoxicology: a review. Environ Mol Mutagen 34:246–255
Fairbairn DW, Olive PL, O’Neill KL (1995) The comet assay: a comprehensive review. Mutat Res 339:37–59
Farah MA, Bushra A, Niamat AM, Rubina S, Waseem A (2004) Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere 55:257–265
Fellers GM, McConnell LL, Pratt D, Datta S (2004) Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada mountains of California, USA. Environ Toxicol Chem 23:2170–2177
Geng BR, Yao D, Zhang JQ, Huang H (2005) Toxicity influence of dichlorvos and butachlor on Rana guentheri tadpoles. China Environ Sci 25(suppl):118–121
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Guo XJ (2006) Product registration situation of chlorimuron-ethyl. New Century Agrochem 6:20
Hu XX (1998) The current situation and developing trends of pesticide industry in China. Pesticides 37:7–10
Huang M, Liu ZJ, Cai ZM (2005) Determination of residue amount of bensulfuron-methyl, metsulfuron-methyl and acetochlor in paddyfield water by HPLC J Hunan Agri Univ (Natural Sciences) 31:213–215
Lajmanovich RC, Sánchez-Hernández JC, Stringhini G, Peltzer PM (2002) First registrations of oganochlorine pesticides residues in amphibians of the Mesopotanmic region, Argentina. Froglog 54:4–5
Lin MF, Wu CL, Wang TC (1987) Pesticide clastogenicity in Chinese hamster ovary cells. Mutat Res 188:241–250
Liu M, Wang TB, Shen XD (2005a) Market and technical process of acetochlor in China. Chem Techno-Econ 23:14–16
Liu SY, Chen YP, Yu HQ, Zhang SJ (2005b) Kinetics and mechanisms of radiation-induced degradation of acetochlor. Chemosphere 59:13–19
Loman J, Andersson G (2007) Monitoring brown frogs Rana arvalis and Rana temporaria in 120 southern Swedish ponds 1989–2005, Mixed trends in different habitats. Biol Conserv 135:46–56
Lowcock LA, Sharbel TF, Bonin J, Ouellet M, Rodrigue J, Des Granges JL (1997) Flow cytometric assay for in vivo genotoxic effects of pesticides in Green frogs (Rana clamitans). Aquat Toxicol 38:241–255
Mitchelmore CL, Chipman JK (1998) DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res 399:135–147
Osano O, Admiraal W, Otieno D (2002) Developmental disorders in embryos of the frog Xenopus laevis induced by chloroacetanilide herbicides and their degradation products. Environ Toxicol Chem 21:375–379
Pavlica M, Klobucar GIV, Mojas N, Erben R, Papes D (2001) Detection of DNA damage in haemocytes of zebra mussel using comet assay. Mutat Res 490:209–214
PEDB (2000) Pesticide Ecotoxicity Database. Environmental fate and effects division. In: Office of pesticide programs, US, EPA, Washington, DC pp 344
Phillips HJ (1973) Dye exclusion tests for cell viability. In: Kruse PF, Patterson MJ (eds) Tissue culture: methods and applications. Academic Press, New York, pp 406–408
Pollet I, Li B-Y (2000) Amphibians as indicators of wetland quality in wetlands form from oil sands effluent. Environ Toxicol Chem 19:2589–2597
Rios AC, Salvadori DM, Oliveira SV, Ribeiro LR (1995) The action of the herbicide paraquat on somatic and germ cells of mice. Mutat Res 328:113–118
Rosenstein BS, Chao CKC (1985) Characterization of DNA repair in a mutant cell line derived from ICR 2A frog cells that are hypersensitive to non-dimer DNA damage induced by solar ultraviolet radiation. Mutat Res 146:191–196
Salam AE, Hussein EHA, El-Itriby HA, Anwar WA, Mansour SA (1993) The mutagenicity of Gramoxone (paraquat) on different eukaryotic systems. Mutat Res 319:89–101
Schuytema GS, Nebeker AV (1999) Effects of ammonium nitrate, sodium nitrate, and urea on red-legged frogs, Pacific treefrogs, and African clawed frogs. Bull Environ Contam Toxicol 18:357–364
Sharbel WM (2004) Monitoring DNA damage following radiation exposure using cytokinesis – block micronucleus method and alkaline single-cell gel electrophoresis. Clin Chim Acta 347:15–24
Shi SD, Shao C (2006) A study on the toxicity of two kinds of herbicides to tadpole of Rana rugulosa. Bull Sci Technol 22:173–175
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of damage in individual cells. Exp Cell Res 175:184–191
Sinha S (1995) Genotoxicity of the herbicide butachlor in cultured human lymphocytes. Mutat Res 344:63–67
Steinert SA (1999) DNA damage as a bivalve biomarker. Biomarkers 4:492–496
Sundaram KMS (1995) Distribution, persistence and fate of mexacarbate in the aquatic environment of a mixed-wood boreal forest. J Environ Sci Health (B) 30:651–683
Svendsen C, Spurgeon DJ, Hankard PK, Weeks JM (2004) A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotoxicol Environ Saf 57:20–29
Takizawa M, Komori K, Tampo Y, Yonaha M (2007) Paraquat-induced oxidative stress and dysfunction of cellular redox systems including antioxidative defense enzymes glutathione peroxidase and thioredoxin reductase. Toxicol In Vitro 21:355–363
Vaishampayan A (1985) Mutagenic activity of alachlor, butachlor and carbaryl to a N sub (2)-fixing cyanobacterium Nostoc muscorum. J Agric Sci 104:571–576
Wang TC, Lee TC, Lin MF, Lin SY (1987) Induction of sister-chromatid exchanges by pesticides in primary rat tracheal epithelial cells and Chinese hamster ovary cells. Mutat Res 188:311–321
Yang L, Zhang YM, Liu JH, Huang DJ (2006) The role of reactive oxygen species in the herbicide acetochlor-induced DNA damage on Bufo taddei tadpole liver. Aquat Toxicol 78:21–26
Yao B, Xu JM, Zhuang CL (2003) Behavior of herbicide butachlor in environment. Ecol Environ 12:66–70
Zaruk D, Alaee M, Sverko E, Comba M (1998) Occurrence of triazine herbicides and metolachlor in the Niagara River and other major tributaries draining into Lake Ontario. Anal Chim Acta 376:113–117
Zhang ZZ, Heng ZC, Li R, Tan ZQ (1998) Study of optimum condition on single cell gel electrophoresis assay. J Health Toxicol 12:249–251
Zheng HH, Ye CM (2001) Analysis of acetochlor and butachlor residues in environmental samples. China Environ Sci 21:21–220
Acknowledgements
The authors gratefully acknowledge the support of National Natural Science Foundation of China (20677008). We thank Mr. Zheng Xiaojun (Senior Experimentalist) and Dr. Wu Huiming for helping with operating the instrument used.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, X.H., Li, S.N., Zhang, L. et al. Evaluation of DNA damage in Chinese toad (Bufo bufo gargarizans) after in vivo exposure to sublethal concentrations of four herbicides using the comet assay. Ecotoxicology 17, 280–286 (2008). https://doi.org/10.1007/s10646-008-0195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0195-z