Abstract
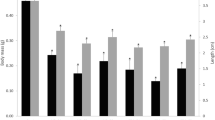
Phenanthrene (PHE), a tricyclic polycyclic aromatic hydrocarbon (PAH), is ubiquitously found in aquatic environments. It is one of the major components in PAH mixtures. It has been identified as one of the 16 priority PAHs for toxicological evaluations. PHE is reported to induce lethal and sub-lethal toxicity in various aquatic indicator organisms. However, no toxicological data of PHE in anuran amphibians could be found. Amphibian larvae (tadpoles) develop in aquatic habitats. Therefore, exposure to PHE could negatively impact their development and fitness in later periods as they move in to the terrestrial habitat following metamorphosis. In the present study, we have analyzed the effects of PHE in Euphlyctis cyanophlyctis tadpoles. PHE induced concentration-dependent lethal effects in the tadpoles. The estimated LC50 values were 16.52, 15.29, 13.69, and 12.28 mg/L at 24, 48, 72, and 96 h of exposure respectively. These LC50 values are significantly higher than the reported environmental concentration of PHE. However, the strong negative correlation (R2 = 0.997, p < 0.001) between the LC50 value and exposure time indicates that longer exposure to lower concentration may cause significant lethal effects. Besides, PHE at environmentally relevant concentrations induced significant sub-lethal toxicities. Exposure to sub-lethal concentrations was found to be genotoxic in erythrocyte micronucleus as well as comet assays. Sub-lethal concentrations of PHE significantly increased superoxide dismutase activity and tissue glutathione level as well as induced lipid peroxidation. The present findings clearly indicate that PHE is a potential threat to the early life stages of amphibians. Further investigations are necessary to ascertain the implications of these early effects during adult life stages.




Similar content being viewed by others
References
Aas E, Baussant T, Balk L, Liewenborg B, Andersen OK (2000) PAH metabolites in bile, cytochrome P4501A and DNA adducts as environmental risk parameters for chronic oil exposure: a laboratory experiment with Atlantic cod. Aquat Toxicol 51(2):241–258
Abdel-Shafy AI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyptian J Petroleum 25(1):107–123
Anyakora C, Ogbeche A, Palmer P, Coker H (2005) Determination of polynuclear aromatic hydrocarbons in marine samples of Siokolo fishing settlement. J Chromatogr A 1073(1–2):323–330
ATSDR (1995) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). Agency for Toxic Substances and Disease Registry, Atlanta
Ball A, Truskewycz A (2013) Polyaromatic hydrocarbon exposure: an ecological impact ambiguity. Environ Sci Pollut Res Int 20(7):4311–4326
Barni S, Boncompagni E, Grosso A, Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54
Bhagat J, Sarkar A, Ingole BS (2016) DNA damage and oxidative stress in marine gastropod Morula granulata exposed to Phenanthrene. Water Air Soil Pollut 227:114
Boone MD, Bridges CM (2003) Effects of carbaryl on green frog (Rana clamitans) tadpoles: timing of exposure versus multiple exposures. Environ Toxicol Chem 22(11):2695–2702
Correia AD, Goncalves R, Scholze M, Ferreira M, Reis-Henriques MA (2007) Biochemical and behavioral responses in gilthead seabream (Sparus aurata) to phenanthrene. J Exp Mar Biol Ecol 347(1–2):109–122
Djomo JE, Ferrier V, Gauthier L, Zoll-Moreux C, Marty J (1995) Amphibian micronucleus test in vivo: evaluation of the genotoxicity of some major polycyclic aromatic hydrocarbons found in a crude oil. Mutagenesis 10(3):223–226
Duke O (2008) Source determination of polynuclear aromatic hydrocarbons in water and sediment of a creek in the Niger Delta region. African J Biotech 7(3):228–285
Fanali LZ, Franco-Belussi L, Bonini-Domingos CR, de Oliveira C (2018) Effects of benzo[a]pyrene on the blood and liver of Physalaemus cuvieri and Leptodactylus fuscus (Anura: Leptodactylidae). Environ Pollut 237:93–102
Forman HJ, Augusto O, Brigelius-Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts LJ, Vina J, Davies KJ (2015) Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic Biol Med 78:233–235
Giri A, Giri S (2019). Micronucleus assays in amphibians. In: The Micronucleus Assay in Toxicology.Siegfried Knasmüller and Michael Fenech (Eds.); Issues in Toxicology No. 39; The Royal Society of Chemistry; 259–272pp. https://doi.org/10.1039/9781788013604-00259
Giri A, Yadav SS, Giri S, Sharma GD (2012) Effect of predator stress and malathion on tadpoles of Indian skittering frog. Aquat Toxicol 106-107:157–163
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16(3):183–190
Hannam ML, Bamber SD, Galloway TS, Moody AJ, Jones MB (2010) Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 78(7):779–784
Hersikorn BD, Smits JEG (2011) Compromised metamorphosis and thyroid hormone changes in wood frogs (Lithobates sylvaticus) raised on reclaimed wetlands on the Athabasca oil sands. Environ Pollut 159(2):596–601
Hiller E, Zemanova L, Sirotiak M, Jurkovic L (2011) Concentrations, distributions, and sources of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in bed sediments of the water reservoirs in Slovakia. Environ Monit Assess 173(1–4):883–897
Hopkins WA (2007) Amphibians as models for studying environmental change. ILAR J 48(3):270–277
Hwang HM, Foster GD (2006) Characterization of polycyclic aromatic hydrocarbons in urban stormwater runoff flowing into the tidal Anacostia River, Washington, DC, USA. Environ Pollut 140:416–426
IARC (1983) Polynuclear aromatic compounds part I: chemical, environmental and experimental data. International Agency for Research on Cancer, Lyon
Incardona JP, Day HL, Collier TK, Scholz NL (2006) Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol 217(3):308–321
IPCS (1998) Selected non-heterocyclic polycyclic aromatic hydrocarbons. Environmental Health Criteria 202. International Programme on Chemical Safety, World Health Organization, Geneva
Jiao H, Wang Q, Zhao N, Jin B, Zhuang X, Bai Z (2017) Distributions and sources of polycyclic aromatic hydrocarbons (PAHs) in soils around a chemical plant in Shanxi, China. Int J Environ Res Public Health 14(10):1198
Khan MI, Cheema SA, Tang X, Hashmi MZ, Shen C, Park J, Chen Y (2013) A battery of bioassays for the evaluation of phenanthrene biotoxicity in soil. Arch Environ Contam Toxicol 65(1):47–55
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollut Bull 42(8):656–666
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30
Machado AA, Hoff ML, Klein RD, Cordeiro GJ, Lencina Avila JM, Costa PG, Bianchini A (2014) Oxidative stress and DNA damage responses to phenanthrene exposure in the estuarine guppy Poecilia vivipara. Marine Environ Res 98:96–105
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Martins M, Ferreira AM, Costa MH, Costa PM (2016) Comparing the genotoxicity of a potentially carcinogenic and a noncarcinogenic PAH, singly, and in binary combination, on peripheral blood cells of the European sea bass. Environ Toxicol 31(11):1307–1318
Michel C, Vincent-Hubert F (2015) DNA oxidation and DNA repair in gills of zebramussels exposed to cadmium and benzo (a) pyrene. Ecotoxicology 24:2009–2016
Mitchelmore CL, Birmelin C, Chipman JK, Livingstone DR (1998) Evidence for cytochrome P-450 catalysis and free radical involvement in the production of DNA strand breaks by benzo[a]pyrene and nitroaromatics in mussel (Mytilus edulis L.) digestive gland cells. Aquat Toxicol. 41(3):193–212
Montalvão MF, Malafaia G (2017) Effects of abamectin on bullfrog tadpoles: insights on cytotoxicity. Environ Sci Pollut Res Int 24(29):23411–23416
Morse HR, Jones NJ, Peltonen K, Harvey RG, Waters R (1996) The identification and repair of DNA adducts induced by waterborne benzo [a] pyrene in developing Xenopus laevis larvae. Mutagenesis 11:101–109
Oliveira M, Pacheco M, Santos MA (2007) Cytochrome P4501A, genotoxic and stress responses in golden grey mullet (Liza aurata) following short-term exposure to phenanthrene. Chemosphere 66(7):1284–1291
Oliveira M, Pacheco M, Santos MA (2008) Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. Sci Total Environ 396(1):70–78
Palanikumar L, Kumaraguru AK, Ramakritinan CM, Anand M (2012) Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol Environ Saf 75:187–197
Patar A, Giri A, Boro F, Bhuyan K, Singha U, Giri S (2016) Cadmium pollution and amphibians - studies in tadpoles of Rana limnocharis. Chemosphere 144:1043–1049
Pérez-Iglesias JM, Brodeur JC, Larramendy ML. (2019) An imazethapyr-based herbicide formulation induces genotoxic, biochemical, and individual organizational effects in Leptodactylus latinasus tadpoles (Anura: Leptodactylidae). doi: https://doi.org/10.1007/s11356-019-06880-7
Pollo FE, Grenat PR, Salinas ZA, Otero MA, Salas NE, Martino AL (2017) Evaluation in situ of genotoxicity and stress in south American common toad Rhinella arenarum in environments related to fluorite mine. Environ Sci Pllut Res Int 24(22):18179–18187
Regnault C, Worms IA, Oger-Desfeux C, MelodeLima C, Veyrenc S, Bayle ML, Combourieu B, Bonin A, Renaud J, Raveton M, Reynaud S (2014) Impaired liver function in Xenopus tropicalis exposed to benzo[a]pyrene: transcriptomic and metabolic evidence. BMC Genomics 15:666
Rehman SU (1984) Lead induced regional lipid peroxidation in brain. Toxicol Lett 21:333–337
Sedlak J, Lindsay RH (1968) Estimation of total protein-bound and nonprotein sulfhydryl groups in tissues with Ellman’s reagent. Anal Biochem 24:192–205
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175(1):184–191
Singha U, Pandey N, Boro F, Giri A, Giri S, Biswas S (2014) Sodium arsenite induced changes in survival, growth, metamorphosis and genotoxicity in the Indian cricket frog (Rana limnocharis). Chemosphere 112:333–339
Sogbanmu TO, Nagy E, Phillips DH, Arlt VM, Otitoloju AA, Bury NR (2016) Lagos lagoon sediment organic extracts and polycyclic aromatic hydrocarbons induce embryotoxic, teratogenic and genotoxic effects in Danio rerio (zebrafish) embryos. Environ Sci Pollut Res Int 23:14489–14501
Stowers SJ, Anderson MW (1985) Formation and persistence of benzo(a)pyrene metabolite-DNA adducts. Environ Health Perspect 62:31–39
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306(5702):1783–1786
Stuart SN, Hoffmann M, Chanson JS, Cox NA, Berridge RJ, Ramani P, Young BE (2008) Threatened amphibians of the world. Lynx Edicions, Barcelona, Spain; IUCN, gland, Switzerland; and Conservation International, Arlington, Virginia, USA. PP.776
Sun Y, Yu H, Zhang J, Yin Y, Shi H, Wang X (2006) Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere 63(8):1319–1327
Sun J, Ni H, Zeng H (2012) Ecological risk assessment of parent and halogenated polycyclic aromatic hydrocarbons in surface sediments from an urban river in south China. Environ Toxicol Chem 31(8):1867–1873
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
USACHPPM (2006) Wildlife toxicity assessment for Phenanthrene. U.S. Army Center for health promotion and preventive medicine (USACHPPM) project number 39-EJ1138-01K, Aberdeen proving ground, Maryland
USEPA (2002) Methods for measuring the acute toxicity of effluents and receiving Waters to freshwater and marine organisms, fifth edition. U.S. Environmental Protection Agency Office of water (4303T) 1200 Pennsylvania avenue, NW Washington, DC 20460, EPA-821-R-02-012
Vera Candioti J, Soloneski S, Larramendy ML (2010) Genotoxic and cytotoxic effects of the formulated insecticide Aficida® on Cnesterodon decemmaculatus (Jenyns, 1842) (Pisces: Poeciliidae). Mutat Res 703:180–186
Vrana B, Paschke A, Popp P (2001) Polyaromatic hydrocarbon concentrations and patterns in sediments and surface water of the Mansfeld region, Saxony-Anhalt, Germany. J Environ Monit 3:602–609
Wang P, Luo L, Ke L, Luan T, Tam NF (2013) Combined toxicity of polycyclic aromatic hydrocarbons and heavy metals to biochemical and antioxidant responses of free and immobilized selenastrum capricornutum. Environ Toxicol Chem 32(3):673–683
Willens S, Stoskopf MK, Baynes RE, Lewbart GA, Taylor SK, Kennedy-Stoskopf S (2006) Percutaneous malathion absorption by anuran skin in flow-through diffusion cells. Environ Toxicol Pharmacol 22(3):255–262
Wu YL, Wang XH, Li YY, Hong HS (2011) Occurrence of polycyclic aromatic hydrocarbons (PAHs) in seawater from the Western Taiwan Strait, China. Mar Pollut Bull 63(5–12):459–463
Yadav SS, Giri S, Singha U, Boro F, Giri A (2013) Toxic and genotoxic effects of roundup on tadpoles of the Indian skittering frog (Euflictis cyanophlyctis) in the presence and absence of predator stress. Aquat Toxicol 132-133:1–8
Yin Y, Jia H, Sun Y, Yu H, Wang X, Wu J, Xue Y (2007) Bioaccumulation and ROS generation in liver of Carassius auratus, exposed to phenanthrene. Comp Biochem Physiol Part C 145(2):288–293
Zhang Y, Tao S (2009) Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos Environ 43(4):812–819
Acknowledgments
The efforts of Indranil Das for reviewing the manuscript are thankfully acknowledged. The infrastructural support provided under DST-FIST program by Govt. of India is thankfully acknowledged.
Funding
The authors, namely, AG and KB, acknowledge the fellowship grants received from the Department of Biotechnology, Govt. of India, under Twinning program. AP acknowledges the financial assistance received under National fellowship for higher education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care was taken in accordance with Institutional Ethical Guidelines with the approval number IEC-AUS/2015-032.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 89 kb)
Rights and permissions
About this article
Cite this article
Bhuyan, K., Patar, A., Singha, U. et al. Phenanthrene alters oxidative stress parameters in tadpoles of Euphlyctis cyanophlyctis (Anura, Dicroglossidae) and induces genotoxicity assessed by micronucleus and comet assay. Environ Sci Pollut Res 27, 20962–20971 (2020). https://doi.org/10.1007/s11356-020-08609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08609-3