Summary
Introduction. The combination of BRAF and MEK inhibitors has deeply changed the treatment of BRAF V600-mutant non-small cell lung cancer patients. These agents demonstrated high antitumor activity as well as safe and manageable toxicity profile. Hypertension, pyrexia and increased liver enzymes are the most common adverse events. Gastrointestinal toxicities are rare, and mainly consist of mild grade vomiting and diarrhea. Case report. We report the case of 70-year-old man affected by BRAF V600-mutant NSCLC with bilateral lung and bone metastases. First-line treatment with encorafenib (450 mg once daily) and binimetinib (45 mg twice daily) was administered within a clinical trial. At the first radiological assessment, computed tomography (CT) scan showed a partial response and signs of intestinal inflammation were reported. The investigational treatment was timely withheld. The subsequent colonoscopy demonstrated the presence of ulcerative lesions at the caecal tract, and the histological diagnosis suggested a drug-induced colitis. No specific treatment was given as the patient did not report abdominal disturbances. Forty-five days after treatment interruption a new CT scan showed the resolution of bowel inflammation and investigational treatment was resumed at the same doses. The patient is still alive and free of toxicity recurrence after 11 months from treatment initiation. Conclusion. Severe gastrointestinal toxicities are uncommon with BRAF and MEK inhibitors, although cases of colitis and intestinal perforation have already been reported in literature. The pathogenesis seems to be related to the MAPK pathway inhibition performed by MEK inhibitors. These adverse events should be accounted given the potential to evolve into life-threatening conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRAF gene mutations can be detected in approximately 2–4% of advanced non-small cell lung cancer (NSCLC) patients. About half of them are V600E mutations that determine the constitutive activation of the BRAF kinase domain, leading to cancer growth, proliferation and survival [1, 2]. Recently, the evidence of high antitumor activity as well as a safe and manageable toxicity profile of dabrafenib, a BRAF inhibitor, and trametinib, a MEK inhibitor, allowed this combination to become a new standard-of-care for BRAF V600-mutant NSCLC patients [3, 4]. Novel combinations of BRAF and MEK inhibitors, such as encorafenib and binimetinib, are under evaluation (NCT03915951). Liver function tests and creatine phosphokinase increase, hypertension and pyrexia are the most frequently reported grade ≥ 3 adverse events. Severe gastrointestinal toxicities, mostly abdominal pain, diarrhea and vomiting, had low incidence in the clinical trials testing these agents [3, 4].
Case report
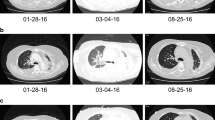
Herein, we report our experience with a 70-year-old man diagnosed with a BRAFV600E-mutant, PD-L1 positive (tumor proportion score 90%) adenocarcinoma of the lung. Baseline CT scan showed bilateral lung lesions and bone dissemination. The patient received the combination of encorafenib (450 mg once daily) and binimetinib (45 mg twice daily) as upfront treatment within a clinical trial (NCT03915951). Two months after starting treatment the radiological assessment showed a partial response (45% decrease of target lesions per RECIST 1.1). As incidental finding, a contrast-enhanced increased thickness of the last ileal loop associated with perivisceral fat suffusion and enlarged lymph nodes was detected (Fig. 1a). Baseline CT scans were reviewed and no inflammatory finding was detected at that level. The treatment was withheld in suspected bowel inflammatory disease and a colonoscopy was performed. The intestinal endoscopy showed a diffuse mucosal erythema of the right upper colon and the presence of two ulcerative lesions at the caecal tract (Fig. 2a). The pathologic examination showed a mixed inflammatory infiltrate in the lamina propria of the caecum, associated with severe eosinophilia. Neither intraepithelial lymphocytes nor epithelial apoptotic bodies, known to be related to immune-mediated damage, were detected (Fig. 2b-d) and CD4/CD8 ratio was more than 1. All these findings suggested a diagnosis of drug-induced colitis.
A 7-cm long contrast-enhanced marked thickening of the last ileal tract, along with the involvement of caecum and appendix, perivisceral adipose tissue suffusion and multiple enlarged lymph nodes at CT scan carried out 2 months after the beginning of BRAF/MEK TKIs (a); almost complete remission of previous radiological findings at CT scan performed after 1 month from treatment interruption (b)
Endoscopic imaging showing the largest ulcerative lesion (1.5 cm) of the caecum with fibrinous and granulation tissue, as per reparative processes (a); corresponding microscopic examination at 10x (b), 20x (c) and 40x (d) magnification. The glands are normally oriented, with a marked chronic inflammatory infiltrate and eosinophils (more than 60/40x, see arrow). Note the increased eosinophils (more than 60/40x) both in the lamina propria and within the glands (arrow). These features are associated with drug-induced mucosal eosinophilia
No specific treatment was given as the patient did not report abdominal disturbances. Based on the evidence of complete recovery of prior radiological findings at a CT scan (Fig. 1b) performed 45 days after treatment interruption, the investigational drugs were resumed at the same doses. The patient is still alive 11 months after the start of treatment and he continues to take encorafenib and binimetinib at full doses with no evidence of toxicity recurrence.
Discussion
Diarrhea and vomiting were the most frequent grade ≥ 3 gastrointestinal toxicities in the clinical trials testing BRAF inhibitors alone or combined with MEK inhibitors [3,4,5]. Rare cases of colitis and intestinal perforation have been reported [6,7,8,9]. The Ras-MEK-ERK pathway plays a crucial role in the proliferation, differentiation, migration and survival of the gastrointestinal epithelium. The underlying mucosal damage that causes colitis might be related to the inhibition of this signalling pathway by MEK inhibitors [10].
These adverse events seem to have a higher incidence combining BRAF and MEK inhibitors than to BRAF inhibitors alone. A correct management of these treatment-related adverse events requires the treatment withdrawal, that will be resumed at reduced dose at the resolution of the gastrointestinal toxicity [11]. Mourad et al. [6] conducted a retrospective analysis of severe gastrointestinal toxicities in melanoma patients treated with BRAF and MEK inhibitors alone or combined. They described three cases of colitis (2 of them treated with BRAF and MEK inhibitors and 1 treated with MEK inhibitor alone), all of them presenting as watery diarrhea. Colitis resolved following treatment withdrawal, and contrary to the management of our patient the MEK inhibitor was not resumed. Moreover, two patients treated with BRAF and MEK inhibitors developed intestinal perforation that required urgent surgical management, leading to a permanent ileostomy in one case. Other cases of intestinal perforation associated to MEK inhibitors have been described, although in some cases tumor regression in response to treatment may be the underlying cause of the event itself [7,8,9].
Notably, we initially assumed that our case was an immune-mediated colitis. However, following an accurate histological examination including immune cell staining and CD4/CD8 ratio, it was excluded as the main pathological mechanism. In fact, immune related adverse events (irAEs), which have been typically described in patients treated with immune-checkpoint inhibitors, are an emerging type of toxicity associated with BRAF and MEK inhibitors [12]. Ben-Betzalel and colleagues described possible irAEs developing in 10 patients on BRAF ± MEK inhibitors. The immune-mediated mechanism was supposed due to the nature of the event (vitiligo, uveitis, erythema nodosum and keratitis sicca), as confirmatory biopsies were not performed [12]. Patients who developed possible irAEs showed higher response rate, deeper tumor responses and prolonged progression-free survival. In light of this finding, it is important to accurately characterize suspected irAEs given the prognostic role that these may retain.
In conclusion, the risk of developing colitis should be accounted in patients treated with BRAF and MEK inhibitors, as it represents an uncommon adverse event with the potential to evolve into life-threating conditions, such as intestinal perforation. A timely and accurate histopathological characterization of the lesion might provide relevant prognostic and therapeutic implications.
Data availability
The current research was entirely conducted in our Institution.
Code availability
Not applicable.
References
Barlesi F, Mazieres J, Merlio JP et al (2016) for the Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 3871415–26
Paik PK, Arcila ME, Fara M et al (2011) Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 29:2046–2051
Planchard D, Smit EF, Groen HJM et al (2017) Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18(10):1307–1316
Planchard D, Besse B, Groen HJM et al (2016) Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016 Jul;17(7):984–993. Epub 2016 Jun 6. PMID: 27283860; PMCID: PMC4993103. https://doi.org/10.1016/S1470-2045(16)30146-2
Ascierto PA, Schadendorf D, Berking C et al (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 14(3):249–256. https://doi.org/10.1016/S1470-2045(13)70024-X
Mourad N, Lourenço N, Delyon J et al (2019) Severe gastrointestinal toxicity of MEK inhibitors. Melanoma Res 29(5):556–559. https://doi.org/10.1097/CMR.0000000000000618
Kass SL, Linden AF, Jackson PG, De Brito PA, Atkins MB (2015) Bowel perforation associated with robust response to BRAF/MEK inhibitor therapy for BRAF-mutant melanoma: a case report. Melanoma Manag 2:115–120. https://doi.org/10.2217/mmt.15.10
Uppaluri R, Winkler AE, Lin T et al (2017) Biomarker and tumor responses of oral cavity squamous cell carcinoma to trametinib: a phase II neoadjuvant window-of-opportunity clinical trial. Clin Cancer Res 23:2186–2194. https://doi.org/10.1158/1078-0432.CCR-16-1469
Shimada Y, Sato Y, Tachikawa R, Hara S, Tomii K (2021) Gastrointestinal perforation following dabrafenib and trametinib administration in non-small cell lung carcinoma with BRAF V600E mutation: a case report and literature review [published online ahead of print, 2021 May 23]. Invest New Drugs. https://doi.org/10.1007/s10637-021-01135-0
Osaki LH, Gama P (2013) MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int J Mol Sci 14:10143–10161
Greco A, Safi D, Swami U, Ginader T, Milhem M, Zakharia Y (2019) Efficacy and Adverse Events in Metastatic Melanoma Patients Treated with Combination BRAF Plus MEK Inhibitors Versus BRAF Inhibitors: A Systematic Review. Cancers (Basel). 2019;11(12):1950. Published 2019 Dec 5. https://doi.org/10.3390/cancers11121950
Ben-Betzalel G, Baruch EN, Boursi B et al (2018) Possible immune adverse events as predictors of durable response to BRAF inhibitors in patients with BRAF V600-mutant metastatic melanoma. Eur J Cancer 101:229–235. https://doi.org/10.1016/j.ejca.2018.06.030
Acknowledgements
The authors thank Steering Committee members of the PHAROS Study for their comments to the draft of this paper
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. No source of funding supported the preparation of the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
The patient declared to consent to participate in this study.
Consent for publication
The patient declared to consent to the publication of the current paper.
Conflicts of interest
Francesco Gelsomino received honoraria for advisory board participation: Eli-Lilly. Andrea Ardizzoni received honoraria for advisory board participation: BMS, MSD, ROCHE, Astra Zeneca, Eli-Lilly. Research Grants: Celgene, BMS, Ipsen, Roche. All remaining authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelsomino, F., Di Federico, A., Tardio, M.L. et al. Drug-induced colitis on BRAF and MEK inhibitors for BRAF V600E-mutated non-small cell lung cancer: a case report. Invest New Drugs 40, 190–193 (2022). https://doi.org/10.1007/s10637-021-01166-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01166-7