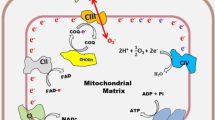
Summary
Ferroptosis is a type of oxidative stress-dependent regulated necrosis characterized by excessive lipid peroxide accumulation. This novel cell death modality has been implicated in preventing cancer progression. Cancer cells tend to modulate their redox state to prevent excessive peroxidation, eventually facilitating tumor growth. System Xc− (a cystine/glutamate antiporter system) is a promising target in cancer cells for ferroptosis induction. The overexpression of system Xc−, especially its core subunit xCT, has been reported in several tumors, and these high expression levels were closely related to cancer cell proliferation, invasion, metastasis and the tumor microenvironment. xCT might serve as a novel biomarker, and its upregulation almost always indicates drug tolerance and poor survival. Therefore, system Xc− inhibition may enhance chemotherapy sensitivity and optimize patient prognosis. Here, we elaborate on the mediation of ferroptosis by suppressing system Xc− and the relevant underlying molecular mechanism in cancer cells. The spotlight on this approach to cancer treatment is creating a new horizon and pointing to future opportunities.

Similar content being viewed by others
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Dixon SJ (2017) Ferroptosis: bug or feature? Immunol Rev 277(1):150–157. https://doi.org/10.1111/imr.12533
Fearnhead HO, Vandenabeele P, Vanden Berghe T (2017) How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ 24(12):1991–1998. https://doi.org/10.1038/cdd.2017.149
Liang C, Zhang X, Yang M, Dong X (2019) Recent progress in ferroptosis inducers for cancer therapy. Adv Mater 31(51):e1904197. https://doi.org/10.1002/adma.201904197
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34(2–3):139–158. https://doi.org/10.1016/j.mam.2012.10.007
Koppula P, Zhang Y, Zhuang L, Gan B (2018) Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun 38(1):12. https://doi.org/10.1186/s40880-018-0288-x
Bridges RJ, Natale NR, Patel SA (2012) System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165(1):20–34. https://doi.org/10.1111/j.1476-5381.2011.01480.x
Polewski MD, Reveron-Thornton RF, Cherryholmes GA, Marinov GK, Cassady K, Aboody KS (2016) Increased expression of system xc− in glioblastoma confers an altered metabolic state and temozolomide resistance. Mol Cancer Res 14(12):1229–1242. https://doi.org/10.1158/1541-7786.MCR-16-0028
Chung WJ, Lyons SA, Nelson GM et al (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25(31):7101–7110. https://doi.org/10.1523/JNEUROSCI.5258-04.2005
Takeuchi S, Wada K, Toyooka T et al (2013) Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery 72(1):33–41. https://doi.org/10.1227/NEU.0b013e318276b2de
Robert SM, Buckingham SC, Campbell SL et al (2015) SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med 7(289):289ra86. https://doi.org/10.1126/scitranslmed.aaa8103
Polewski MD, Reveron-Thornton RF, Cherryholmes GA, Marinov GK, Aboody KS (2017) SLC7A11 overexpression in glioblastoma is associated with increased cancer stem cell-like properties. Stem Cells Dev 26(17):1236–1246. https://doi.org/10.1089/scd.2017.0123
Ma Z, Zhang H, Lian M et al (2017) SLC7A11, a component of cysteine/glutamate transporter, is a novel biomarker for the diagnosis and prognosis in laryngeal squamous cell carcinoma. Oncol Rep 38(5):3019–3029. https://doi.org/10.3892/or.2017.5976
Ji X, Qian J, Rahman SMJ et al (2018) xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 37(36):5007–5019. https://doi.org/10.1038/s41388-018-0307-z
Shin SS, Jeong BS, Wall BA et al (2018) Participation of xCT in melanoma cell proliferation in vitro and tumorigenesis in vivo. Oncogenesis 7(11):86. https://doi.org/10.1038/s41389-018-0098-7
Arensman MD, Yang XS, Leahy DM et al (2019) Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc Natl Acad Sci USA 116(19):9533–9542. https://doi.org/10.1073/pnas.1814932116
Shiozaki A, Iitaka D, Ichikawa D et al (2014) xCT, component of cysteine/glutamate transporter, as an independent prognostic factor in human esophageal squamous cell carcinoma. J Gastroenterol 49(5):853–863. https://doi.org/10.1007/s00535-013-0847-5
Sugano K, Maeda K, Ohtani H, Nagahara H, Shibutani M, Hirakawa K (2015) Expression of xCT as a predictor of disease recurrence in patients with colorectal cancer. Anticancer Res 35(2):677–682
Chen RS, Song YM, Zhou ZY et al (2009) Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 28(4):599–609. https://doi.org/10.1038/onc.2008.414
Wolf IM, Fan Z, Rauh M et al (2014) Histone deacetylases inhibition by SAHA/Vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci Rep 4:6226. https://doi.org/10.1038/srep06226
Ye P, Mimura J, Okada T et al (2014) Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol 34(18):3421–3434. https://doi.org/10.1128/MCB.00221-14
Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N (2017) ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 36(40):5593–5608. https://doi.org/10.1038/onc.2017.146
Habib E, Linher-Melville K, Lin HX, Singh G (2015) Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol 5:33–42. https://doi.org/10.1016/j.redox.2015.03.003
Fan Z, Wirth AK, Chen D et al (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6(8):e371. https://doi.org/10.1038/oncsis.2017.65
Linher-Melville K, Haftchenary S, Gunning P, Singh G (2015) Signal transducer and activator of transcription 3 and 5 regulate system Xc- and redox balance in human breast cancer cells. Mol Cell Biochem 405(1–2):205–221. https://doi.org/10.1007/s11010-015-2412-4
Linher-Melville K, Nashed MG, Ungard RG et al (2016) Chronic inhibition of STAT3/STAT5 in treatment-resistant human breast cancer cell subtypes: Convergence on the ROS/SUMO pathway and its effects on xCT expression and system xc- activity. PLoS One 11(8):e0161202. https://doi.org/10.1371/journal.pone.0161202
Ishimoto T, Nagano O, Yae T et al (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19(3):387–400. https://doi.org/10.1016/j.ccr.2011.01.038
Hasegawa M, Takahashi H, Rajabi H et al (2016) Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 7(11):11756–11769. https://doi.org/10.18632/oncotarget.7598
Ju HQ, Lu YX, Chen DL et al (2016) Redox regulation of stem-like cells though the CD44v-xCT axis in colorectal cancer: mechanisms and therapeutic implications. Theranostics 6(8):1160–1175. https://doi.org/10.7150/thno.14848
Horibe S, Kawauchi S, Tanahashi T, Sasaki N, Mizuno S, Rikitake Y (2018) CD44v-dependent upregulation of xCT is involved in the acquisition of cisplatin-resistance in human lung cancer A549 cells. Biochem Biophys Res Commun 507(1–4):426–432. https://doi.org/10.1016/j.bbrc.2018.11.055
Wang F, Yang Y (2014) Suppression of the xCT-CD44v antiporter system sensitizes triple-negative breast cancer cells to doxorubicin. Breast Cancer Res Treat 147(1):203–210. https://doi.org/10.1007/s10549-014-3068-6
Yoshikawa M, Tsuchihashi K, Ishimoto T et al (2013) xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res 73(6):1855–1866. https://doi.org/10.1158/0008-5472.CAN-12-3609-T
Tsuchihashi K, Okazaki S, Ohmura M et al (2016) The EGF receptor promotes the malignant potential of glioma by regulating amino acid transport system xc(-). Cancer Res 76(10):2954–2963. https://doi.org/10.1158/0008-5472.CAN-15-2121
Suina K, Tsuchihashi K, Yamasaki J et al (2018) Epidermal growth factor receptor promotes glioma progression by regulating xCT and GluN2B-containing N-methyl-d-aspartate-sensitive glutamate receptor signaling. Cancer Sci 109(12):3874–3882. https://doi.org/10.1111/cas.13826
Ranjan A, Iwakuma T (2016) Non-canonical cell death induced by p53. Int J Mol Sci 17(12):2068. https://doi.org/10.3390/ijms17122068
Zhang W, Gai C, Ding D et al (2018) Targeted p53 on small-molecules-induced ferroptosis in cancers. Front Oncol 8:507. https://doi.org/10.3389/fonc.2018.00507
Jiang L, Hickman JH, Wang SJ, Gu W (2015) Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle 14(18):2881–2885. https://doi.org/10.1080/15384101.2015.1068479
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62. https://doi.org/10.1038/nature14344
Gnanapradeepan K, Basu S, Barnoud T et al (2018) The p53 tumor suppressor in the control of metabolism and ferroptosis. Front Endocrinol 9:124. https://doi.org/10.3389/fendo.2018.00124
Wang Y, Yang L, Zhang X et al (2019) Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep 20(7):e47563. https://doi.org/10.15252/embr.201847563
Liu DS, Duong CP, Haupt S et al (2017) Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun 8:14844. https://doi.org/10.1038/ncomms14844
Ye J, Jiang X, Dong Z, Hu S, Xiao M (2019) Low-concentration PTX And RSL3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag Res 11:9783–9792. https://doi.org/10.2147/CMAR.S217944
Saint-Germain E, Mignacca L, Vernier M et al (2017) SOCS1 regulates senescence and ferroptosis by modulating the expression of p53 target genes. Aging 9(10):2137–2162. https://doi.org/10.18632/aging.101306
Chu B, Kon N, Chen D et al (2019) ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21(5):579–591. https://doi.org/10.1038/s41556-019-0305-6
Huang C, Yang M, Deng J et al (2018) Upregulation and activation of p53 by erastin–induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep 40(4):2363–2370. https://doi.org/10.3892/or.2018.6585
Wang Z, Ding Y, Wang X et al (2018) Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett 428:21–33. https://doi.org/10.1016/j.canlet.2018.04.021
Singer E, Judkins J, Salomonis N et al (2015) Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis 6(1):e1601. https://doi.org/10.1038/cddis.2014.566
Daher B, Parks SK, Durivault J et al (2019) Genetic ablation of the cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res 79(15):3877–3890. https://doi.org/10.1158/0008-5472.CAN-18-3855
Okazaki S, Umene K, Yamasaki J et al (2019) Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci 110(11):3453–3463. https://doi.org/10.1111/cas.14182
Lanzardo S, Conti L, Rooke R et al (2016) Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer Res 76(1):62–72. https://doi.org/10.1158/0008-5472.CAN-15-1208
Ruiu R, Rolih V, Bolli E et al (2019) Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol Immunother 68(1):131–141. https://doi.org/10.1007/s00262-018-2185-1
Cobler L, Zhang H, Suri P, Park C, Timmerman LA (2018) xCT inhibition sensitizes tumors to γ-radiation via glutathione reduction. Oncotarget 9(64):32280–32297. https://doi.org/10.18632/oncotarget.25794
Bekeschus S, Eisenmann S, Sagwal SK et al (2020) xCT (SLC7A11) expression confers intrinsic resistance to physical plasma treatment in tumor cells. Redox Biol 30:101423. https://doi.org/10.1016/j.redox.2019.101423
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3(3):285–296. https://doi.org/10.1016/s1535-6108(03)00050-3
Hao S, Yu J, He W et al (2017) Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia (New York) 19(12):1022–1032. https://doi.org/10.1016/j.neo.2017.10.005
Wang H, Liu C, Zhao Y et al (2020) Inhibition of LONP1 protects against erastin-induced ferroptosis in Pancreatic ductal adenocarcinoma PANC1 cells. Biochem Biophys Res Commun 522(4):1063–1068. https://doi.org/10.1016/j.bbrc.2019.11.187
Wang L, Liu Y, Du T et al (2020) ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ 27(2):662–675. https://doi.org/10.1038/s41418-019-0380-z
Shintoku R, Takigawa Y, Yamada K et al (2017) Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci 108(11):2187–2194. https://doi.org/10.1111/cas.13380
Bai T, Liang R, Zhu R et al (2020) MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J Cell Physiol 235(7–8):5637–5648. https://doi.org/10.1002/jcp.29496
Qi W, Li Z, Xia L et al (2019) LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep 9(1):16185. https://doi.org/10.1038/s41598-019-52837-8
Liu N, Lin X, Huang C (2020) Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br J Cancer 122(2):279–292. https://doi.org/10.1038/s41416-019-0660-x
Yang Y, Luo M, Zhang K et al (2020) Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun 11(1):433. https://doi.org/10.1038/s41467-020-14324-x
Sehm T, Rauh M, Wiendieck K et al (2016) Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget 7(46):74630–74647. https://doi.org/10.18632/oncotarget.11858
Gai C, Yu M, Li Z et al (2020) Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J Cell Physiol 235(4):3329–3339. https://doi.org/10.1002/jcp.29221
Zhou HH, Chen X, Cai LY et al (2019) Erastin reverses ABCB1-mediated docetaxel resistance in ovarian cancer. Front Oncol 9:1398. https://doi.org/10.3389/fonc.2019.01398
Dahlmanns M, Yakubov E, Chen D et al (2017) Chemotherapeutic xCT inhibitors sorafenib and erastin unraveled with the synaptic optogenetic function analysis tool. Cell Death Discov 3:17030. https://doi.org/10.1038/cddiscovery.2017.30
Sato M, Kusumi R, Hamashima S et al (2018) The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci Rep 8(1):968. https://doi.org/10.1038/s41598-018-19213-4
Guan J, Lo M, Dockery P, Mahon S et al (2009) The xc- cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol 64(3):463–472. https://doi.org/10.1007/s00280-008-0894-4
Kim EH, Shin D, Lee J, Jung AR, Roh JL (2018) CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett 432:180–190. https://doi.org/10.1016/j.canlet.2018.06.018
Yu H, Yang C, Jian L et al (2019) Sulfasalazine–induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep 42(2):826–838. https://doi.org/10.3892/or.2019.7189
Otsubo K, Nosaki K, Imamura CK et al (2017) Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci 108(9):1843–1849. https://doi.org/10.1111/cas.13309
Yamaguchi Y, Kasukabe T (2018) Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol 52(3):1011–1022. https://doi.org/10.3892/ijo.2018.4259
Wada F, Koga H, Akiba J et al (2018) High expression of CD44v9 and xCT in chemoresistant hepatocellular carcinoma: Potential targets by sulfasalazine. Cancer Sci 109(9):2801–2810. https://doi.org/10.1111/cas.13728
Ogihara K, Kikuchi E, Okazaki S et al (2019) Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci 110(4):1431–1441. https://doi.org/10.1111/cas.13960
Okazaki S, Shintani S, Hirata Y et al (2018) Synthetic lethality of the ALDH3A1 inhibitor dyclonine and xCT inhibitors in glutathione deficiency-resistant cancer cells. Oncotarget 9(73):33832–33843. https://doi.org/10.18632/oncotarget.26112
Otsuki Y, Yamasaki J, Suina K et al (2020) Vasodilator oxyfedrine inhibits aldehyde metabolism and thereby sensitizes cancer cells to xCT-targeted therapy. Cancer Sci 111(1):127–136. https://doi.org/10.1111/cas.14224
Sleire L, Skeie BS, Netland IA et al (2015) Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene 34(49):5951–5959. https://doi.org/10.1038/onc.2015.60
Sehm T, Fan Z, Ghoochani A et al (2016) Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget 7(24):36021–36033. https://doi.org/10.18632/oncotarget.8651
Louandre C, Ezzoukhry Z, Godin C et al (2013) Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer 133(7):1732–1742. https://doi.org/10.1002/ijc.28159
Lachaier E, Louandre C, Godin C et al (2014) Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res 34(11):6417–6422
Dixon SJ, Patel DN, Welsch M et al (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3:e02523. https://doi.org/10.7554/eLife.02523
Sauzay C, Louandre C, Bodeau S et al (2018) Protein biosynthesis, a target of sorafenib, interferes with the unfolded protein response (UPR) and ferroptosis in hepatocellular carcinoma cells. Oncotarget 9(9):8400–8414. https://doi.org/10.18632/oncotarget.23843
Louandre C, Marcq I, Bouhlal H et al (2015) The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett 356(2 Pt B):971–977. https://doi.org/10.1016/j.canlet.2014.11.014
Houessinon A, François C, Sauzay C et al (2016) Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol Cancer 15(1):38. https://doi.org/10.1186/s12943-016-0526-2
Sun X, Niu X, Chen R et al (2016) Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 64(2):488–500. https://doi.org/10.1002/hep.28574
Sun X, Ou Z, Chen R et al (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63(1):173–184. https://doi.org/10.1002/hep.28251
Bai T, Lei P, Zhou H et al (2019) Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med 23(11):7349–7359. https://doi.org/10.1111/jcmm.14594
Roh JL, Kim EH, Jang H, Shin D (2017) Aspirin plus sorafenib potentiates cisplatin cytotoxicity in resistant head and neck cancer cells through xCT inhibition. Free Radic Biol Med 104:1–9. https://doi.org/10.1016/j.freeradbiomed.2017.01.002
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81872080, 81572349), Jiangsu Provincial Medical Talent (ZDRCA2016055), the Science and Technology Department of Jiangsu Province (BK20181148), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the 333 high-level talents of Jiangsu Province (BRA2019083).
Funding
This work was supported by the National Natural Science Foundation of China (No. 81872080, 81572349), Jiangsu Provincial Medical Talent (ZDRCA2016055), the Science and Technology Department of Jiangsu Province (BK20181148), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the 333 high-level talents of Jiangsu Province (BRA2019083).
Author information
Authors and Affiliations
Contributions
MRL and WTZ wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MRL declares that she has no conflict of interest. WTZ declares that he has no conflict of interest. DSP declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Mr., Zhu, Wt. & Pei, Ds. System Xc−: a key regulatory target of ferroptosis in cancer. Invest New Drugs 39, 1123–1131 (2021). https://doi.org/10.1007/s10637-021-01070-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01070-0