Summary
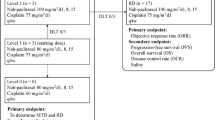
Background A global multicenter study demonstrated superiority of carboplatin + nab-paclitaxel (PTX) therapy compared to carboplatin + PTX in terms of response rate (RR) and non-inferiority in terms of progression free survival (PFS) and overall survival (OS) in untreated patients with stage IIIB/IV non-small cell lung cancer; no clinical findings have so far been reported on maintenance therapies with nab-PTX. The aim of this study was to determine the efficacy and safety of maintenance therapy with nab-PTX following carboplatin + nab-PTX combination therapy. Methods Carboplatin (AUC 6) was administered on Day 1; and nab-PTX 100 mg/m2 on Days 1, 8, and 15, and dosing was repeated in 4 courses of 4 weeks each. In patients with clinical response was observed at the end of the 4th course, nab-PTX maintenance therapy was repeated. Results Out of 39 patients included in the efficacy analysis, 19 (48.7%) patients completed the induction therapy and 15 (38.5%) were transitioned to maintenance therapy. The median PFS in the maintenance phase was 6.5 (90%CI 1.4–11.4) months. The median OS in 15 patients was 12.6 (95%CI: 7.4-not reached). Grade ≥ 3 toxicities observed in more than 5% of patients were neutropenia (55.0%), anemia (15.0%), and febrile neutropenia (5.0%), with no increase during the maintenance phase. Conclusions Although statistically significance was not demonstrated presumably due to a limited transition rate from induction to maintenance phase, nab-PTX was suggested to be a useful treatment option following the induction therapy with nab-PTX in patients with advanced NSCLC.



Similar content being viewed by others
References
Siegel R, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Mon-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594
Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, Nukiwa T, Miyaoka E, Japanese Joint Committee of Lung Cancer Registry (2008) A Japanese lung cancer registry study -prognosis of 13,010 resected lung cancers. J Thorac Oncol 3:46–52
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-arm cooperative study in Japan. Ann Oncol 18:317–323
NSCLC Meta-analyses Collaborative Group (2008) Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 26:4617–4625
Belani CP, Barstis J, Perry M et al (2003) Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol 21:2933–2939
Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, Ciuleanu T, Cucevic B, Gyurkovits K, Ulsperger E, Jassem J, Grgic M, Saip P, Szilasi M, Wiltschke C, Wagnerova M, Oskina N, Soldatenkova V, Zielinski C, Wenczl M, Central European Cooperative Oncology Group CECOG (2006) Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 52:155–163
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomized controlled trial. Lancet Oncol 13:247–255
Westeel V, Quoix E, Moro-Sibilot D, Mercier M, Breton JL, Debieuvre D, Richard P, Haller MA, Milleron B, Herman D, Level MC, Lebas FX, Puyraveau M, Depierre A, for the French Thoracic Oncology Collaborative Group (2005) Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst 97:499–506
Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, Chen R, Hristova-Kazmierski M, Treat J, Obasaju CK, Marciniak M, Gill J, Schiller JH (2009) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 27:591–598
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomized, double-blind, phase 3 study. Lancet 374:1432–1440
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, Ahn MJ, Aerts JGJV, Gorbunova V, Vikström A, Wong EK, Perez-Moreno P, Mitchell L, Groen HJM (2013) Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 31:3004–3011
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, Iglesias JL, Renschler MF (2012) Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 30:2055–2062
Satouchi M, Okammoto I, Sakai H et al (2013) Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer 81:97–101
Barlesi F, Scherpereel A, Gorbunova V, Gervais R, Vikstrom A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M, Pazzola A, Kim HT, Aerts JG, Morando C, Loundou A, Groen HJM, Rittmeyer A (2014) Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 25:1044–1052
Renschler MF (2013) nab-paclitaxel plus carboplatin patients with squamous cell (SCC) non-small cell lung cancer (NSCLC): Analysis of patients treated beyond 4 cycles in pivotal phase III trial. International Association for the Study of lung Cancer (IASCL) Available from URL: https://library.iaslc.org/search-speaker?search_speaker=15899. Accessed 25 Mar 2018
Acknowledgements
Clinical Research Support Center Kyushu, incorporated non-profit organization, supported research at individual study centers, and conducted data collection and data management. We appreciate the assistance by Ms. Ritsuko Kondo for editing the manuscript. The authors thank all the investigators and the patients who participated in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Junji Uchino and Masaki Fujita contributed to the study concept and design, and supervised the study. All authors contributed to acquisition of data. Junji Uchino drafted the report, and all other authors critically reviewed and revised the report for important intellectual content. Junji Kishimoto did the statistical analysis, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflicts of interests
J Uchino reports grants from Eli Lilly Japan K.K., outside the submitted work. T Yamada reports grants from Nippon Boehringer Ingelheim Co., Ltd., and Ono Pharmaceutical Co., Ltd., outside the submitted work. K Takayama reports grants from Chugai-Roche Co., grants from Ono Pharmaceutical Co., personal fees from Astrazeneca Co., personal fees from Chugai-Roche Co., personal fees from MSD-Merck Co., personal fees from Eli Lilly Co., personal fees from Boehringer-Ingelheim Co., personal fees from Daiichi-Sankyo Co., outside the submitted work. A Nakao, F Igata, R On, T Ikeda, H Yatsugi, R Hirano, T Sasaki, K Tanimura, T Imabayashi, N Tamiya, Y Kaneko, N Nagata, K Watanabe, J Kishimoto, and M Fujita have no conflict of interest to declare.
Ethical approval
The present study was conducted in accordance with the Declaration of Helsinki and “Ethical Guidelines for Medical and Health Research Involving Human Subjects”. The study protocol was approved by the ethics committee of each institution participating in this study. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Patients were given written informed consent forms approved by the Ethics Committees, were fully informed about the study in writing, and then freely signed their consent forms.
Rights and permissions
About this article
Cite this article
Nakao, A., Uchino, J., Igata, F. et al. Nab-paclitaxel maintenance therapy following carboplatin + nab-paclitaxel combination therapy in chemotherapy naïve patients with advanced non-small cell lung cancer: multicenter, open-label, single-arm phase II trial. Invest New Drugs 36, 903–910 (2018). https://doi.org/10.1007/s10637-018-0617-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0617-6