Summary
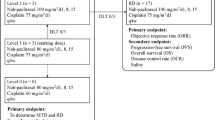
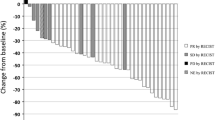
Background This study was designed to determine the recommended dose of a combination of nedaplatin (NED) and nab-paclitaxel (nab-PTX) in chemotherapy-naive patients with advanced squamous non-small-cell lung cancer (NSCLC). Methods Patients received escalating doses of NED on day 1 and nab-PTX on days 1, 8, and 15 every 4 weeks by an intravenous infusion for up to six cycles. Results A dose of 100 mg/m2 NED and 100 mg/m2 nab-PTX was determined to be the recommended dose for patients with advanced squamous NSCLC. The study had an overall response rate of 66.7% (95% confidence interval [CI]: 38.4–88.2) and disease control rate of 93.3% (95% CI: 68.1–99.8). The median progression-free survival time and survival time was 7.0 months (95% CI: 5.9–8.1) and 13.1 months (95% CI: 6.2–20.1), respectively. The most common adverse events were neutropenia (grade 3/4, 33%) and leukopenia (grade 3/4, 27%). Although peripheral neuropathy was observed in 5 patients (grade 1/2), non-hematological toxic effects were relatively mild. Febrile neutropenia, pneumonitis, and treatment-related death were not observed. Conclusions The combination of NED and nab-PTX was a tolerable and effective regimen and its recommended dose was 100 mg/m2 and 100 mg/m2, respectively, in chemotherapy-naive patients with advanced squamous NSCLC (UMIN000010963).


Similar content being viewed by others
References
Devesa SS, Bray F, Vizcaino AP, Parkin DM (2005) International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 117(2):294–299. doi:10.1002/ijc.21183
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98. doi:10.1056/NEJMoa011954
Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF, Israel VK, Livingston RB, Gandara DR (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a southwest oncology group trial. J Clin Oncol 19(13):3210–3218. doi:10.1200/JCO.2001.19.13.3210
Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M (2002) Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 20(21):4285–4291. doi:10.1200/JCO.2002.02.068
Meza R, Meernik C, Jeon J, Cote ML (2015) Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 10(3):e0121323. doi:10.1371/journal.pone.0121323
Sawabata N, Asamura H, Goya T, Mori M, Nakanishi Y, Eguchi K, Koshiishi Y, Okumura M, Miyaoka E, Fujii Y (2010) Japanese lung cancer registry study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 5(9):1369–1375. doi:10.1097/JTO.0b013e3181e452b9
Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, Kazarnowicz A, Park K, Schumann C, Reck M, Depenbrock H, Nanda S, Kruljac-Letunic A, Kurek R, Paz-Ares L, Socinski MA (2015) Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 16(7):763–774. doi:10.1016/S1470-2045(15)00021-2
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. doi:10.1056/NEJMoa061884
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2013) PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31(23):2895–2902. doi:10.1200/JCO.2012.47.1102
Gao G, Ren S, Li A, Xu J, Xu Q, Su C, Guo J, Deng Q, Zhou C (2012) Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: a meta-analysis from six phase III randomized controlled trials. Int J Cancer 131(5):E822–E829. doi:10.1002/ijc.27396
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371(23):2167–2177. doi:10.1056/NEJMoa1408440
Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, Ohe Y, Nogami N, Takeuchi K, Shimada T, Tanaka T, Tamura T (2013) CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 14(7):590–598. doi:10.1016/S1470-2045(13)70142-6
Tabchi S, Kourie HR, Kattan J (2016) Adding checkpoint inhibitors to tyrosine kinase inhibitors targeting EGFR/ALK in non-small cell lung cancer: a new therapeutic strategy. Investig New Drugs 34(6):794–796
Ku BM, Bae YH, Koh J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ (2016) AZD9291 overcomes T790 M-mediated resistance through degradation of EGFR(L858R/T790M) in non-small cell lung cancer cells. Investig New Drugs 34(4):407–415
Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(suppl 7):vii56–vii64. doi:10.1093/annonc/mds226
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) Version 5. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp .Accessed 25 Aug 2015
Japan Lung Cancer Society. Guidelines for the treatment of lung cancer. https://www.haigan.gr.jp/modules/guideline/index.php?content_id=3. Accessed Nov 2016
Suzuki S, Karayama M, Inui N, Fujisawa T, Enomoto N, Nakamura Y, Kuroishi S, Matsuda H, Yokomura K, Koshimizu N, Toyoshima M, Imokawa S, Asada K, Masuda M, Yamada T, Watanabe H, Suda T (2016) Continuation maintenance therapy with S-1 in chemotherapy- naïve patients with advanced squamous cell lung cancer. Investig New Drugs 34(4):490–496
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, Iglesias JL, Renschler MF (2012) Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 30(17):2055–2062. doi:10.1200/JCO.2011.39.5848
Furuse K1, Fukuoka M, Kurita Y, Ariyoshi Y, Niitani H, Yoneda S, Fujii M, Hasegawa K, Nishiwaki Y, Tamura M (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for primary lung cancer. Gan To Kagaku Ryoho 19(6):879–884
Fukuda M, Shinkai T, Eguchi K, Sasaki Y, Tamura T, Ohe Y, Kojima A, Oshita F, Hara K, Saijo N (1990) Phase II study of (glycolate-O, O’) diammineplatinum(II), a novel platinum complex, in the treatment of non-small-cell lung cancer. Cancer Chemother Pharmacol 26(6):393–396
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Shimada M, Itamochi H, Kigawa J (2013) Nedaplatin: a cisplatin derivative in cancer chemotherapy. Cancer Manag Res 8(5):67–76. doi:10.2147/CMAR.S35785
Nakajima O, Inoue S, Kobayashi K (1994) Differences in intracellular uptake of cisplatin, carboplatin and 254-S among human lung cancer cell lines. Japan J Lung Cancer 34:313–319
Naito Y, Kubota K, Ohmatsu H, Goto K, Niho S, Yoh K, Ohe Y (2011) Phase II study of nedaplatin and docetaxel in patients with advanced squamous cell carcinoma of the lung. Ann Oncol 22(11):2471–2475. doi:10.1093/annonc/mdq781
Shukuya T, Yamanaka T, Seto T, Daga H, Goto K, Saka H, Sugawara S, Takahashi T, Yokota S, Kaneda H, Kawaguchi T, Nagase S, Oguri T, Iwamoto Y, Nishimura T, Hattori Y, Nakagawa K, Nakanishi Y, Yamamoto N (2015) Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial. Lancet Oncol 16(16):1630–1638. doi:10.1016/S1470-2045(15)00305-8
Fang Y, Wang L, Xia GH, Shi MQ (2014) Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pac J Cancer Prev 15(17):7453–7457
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA (2002) Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 8(5):1038–1044
Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A, Figg WD (2008) Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 14(13):4200–4205. doi:10.1158/1078-0432
John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD (2003) Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol 284(1):L187–L196. doi:10.1152/ajplung.00152.2002
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res 12(4):1317–1324. doi:10.1158/1078-0432.CCR-05-1634
Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F (2000) Description of a short-term taxol-induced nociceptive neuropathy in rats. Brain Res 887(2):239–249. doi:10.1016/S0006-8993(00)02910-3
Acknowledgements
The authors thank all participants of this study. The authors would like to thank Dr. Akihiro Tamiya (Department of Internal Medicine, Kinki-Chuo Chest Medical Center, Osaka) for evaluating the eligibility, evaluability, and responses in this study. The authors also thank Dr. Seiichiro Kusuhara (Department of Respiratory Medicine, Kitasato University School of Medicine) for collecting the clinical data of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors of this study declare that they have no conflict of interest.
Funding
The work was not supported by any financial support.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Igawa, S., Otani, S., Nakahara, Y. et al. Phase I study of Nedaplatin, a platinum based antineoplastic drug, combined with nab-paclitaxel in patients with advanced squamous non–small cell lung cancer. Invest New Drugs 36, 45–52 (2018). https://doi.org/10.1007/s10637-017-0472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0472-x