Summary
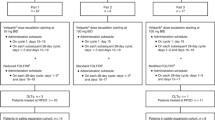
Background The aim of this phase I study was to determine the safety and tolerability and to establish the maximum tolerated dose (MTD) of orally administered olaparib (AZD2281) in combination with topotecan in patients with advanced solid tumors. Patients and methods Patients aged ≥18 years with histologically or cytologically diagnosed advanced solid tumors for whom no suitable effective therapy exists were included. Patients in four cohorts received topotecan (0.5 mg/m2/day × 3 days or 1.0 mg/m2/day × 3 days) intravenously in combination with oral olaparib 50, 100 or 200 mg bid for six cycles. The primary objectives were to determine the safety and tolerability and to establish the MTD of olaparib in combination with topotecan. Results Twenty-one patients were enrolled and 19 received treatment. Dose-limiting toxicities were neutropenia and thrombocytopenia. The MTD was established as topotecan 1.0 mg/m2/day × 3 days plus olaparib 100 mg bid. The most common adverse events (AEs) included fatigue and gastrointestinal events. There was an olaparib and topotecan dose-related increase in neutropenia which was dose limiting. Conclusions Further development of olaparib and topotecan in combination was not explored due to dose-limiting hematological AEs and the resulting sub-therapeutic MTD.

Similar content being viewed by others
References
Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411:366–374
Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429
Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, Javaid H, Kerrigan F, Knights C, Lau A, Loh VM Jr, Matthews IT, Moore S, O'Connor MJ, Smith GC, Martin NM (2008) 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2 H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 51:6581–6591
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O'Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 105:17079–17084
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, Lau A, O'Connor MJ, Jonkers J (2008) Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res 14:3916–3925
Zander SA, Kersbergen A, van de Burg E, de Water N, van Tellingen O, Gunnarsdottir S, Jaspers JE, Pajic M, Nygren AO, Jonkers J, Borst P, Rottenberg S (2010) Sensitivity and acquired resistance of BRCA1; p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res 70:1700–1710
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JHM, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A'Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JHM, de Bono JS, Kaye SB (2010) Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28:2512–2519
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244
Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C (1999) Topotecan - a novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology 56:1–12
ten Bokkel HW, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson N, Spanczynski M, Bolis G, Malmstrom H, Coleman R, Fields SC, Heron JF (1997) Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15:2183–2193
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, Carmichael J, Krebs JB, Ross G, Lane SR, Gralla R (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17:658
Zhao W, Duan W, Leon ME, Chen AP, Sofletea G, Thurmond B, Ramaswamy B, O'Malley D, Bekaii-Saab TS, Villalona-Calero MA (2010) Targeting fanconi anemia (FA) repair pathway deficiency for treatment with PARP inhibitors. J Clin Oncol 28(23s):abst TPS168
Knights C, Chresta C, Riches L, Mangena R, Avis T, O'Shaughnessy A, Unwin L, Dunham V, Jones L, Cranston A, Carmichael J, Martin N, O'Connor M, Lau A (2009) Preclinical evaluation of the PARP inhibitor olaparib in homologous recombination deficient (HRD) MRE11 mutant microsatellite instable (MSI) colorectal cancer. Mol Cancer Ther 8(12 Suppl):A114
Gelmon KA, Hirte HW, Robidoux A, Tonkin KS, Tischkowitz M, Swenerton K, Huntsman D, Carmichael J, MacPherson E, Oza AM (2010) Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple negative breast cancer. J Clin Oncol 28(15S):abst 3002
Borst P, Jonkers J, Rottenberg S (2007) What makes tumors multidrug resistant? Cell Cycle 6:2782–2787
Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A (2008) Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451:1111–1115
Haslam IS, Jones K, Coleman T, Simmons NL (2008) Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem Pharmacol 76:850–861
Schuetz E, Lan L, Yasuda K, Kim R, Kocarek TA, Schuetz J, Strom S (2002) Development of a real-time in vivo transcription assay: application reveals pregnane X receptor-mediated induction of CYP3A4 by cancer chemotherapeutic agents. Mol Pharmacol 62:439–445
Giaccone G, Rajan A, Kelly RJ, Gutierrez M, Kummar S, Yancey M, Ji JJ, Zhang Y, Parchment RE, Doroshow JH (2010) A Phase I combination study of olaparib (AZD2281; KU-0059436) and cisplatin (C) plus gemcitabine (G) in adults with solid tumors. J Clin Oncol 28(15S):abst 3027
Dent RA, Lindeman G, Clemons M, Wildiers H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Kemlsey K, Carmichael J (2010) Safety and efficacy of the oral PARP inhibitor olaparib (AZD2281) in combination with paclitaxel for the 1st or 2nd line treatment of patients with metastatic triple negative breast cancer: results from the safety cohort of a Phase 1/2 multicentre trial. J Clin Oncol 28(15S):abst 1018
O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205–214
Acknowledgements
We thank Dr Juliet Fawcett from Mudskipper who provided editorial assistance funded by AstraZeneca. This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anne Thomas and James Cassidy are joint senior authors.
Rights and permissions
About this article
Cite this article
Samol, J., Ranson, M., Scott, E. et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs 30, 1493–1500 (2012). https://doi.org/10.1007/s10637-011-9682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9682-9