Summary
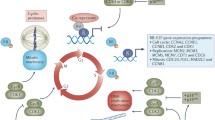
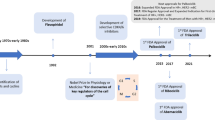
Cyclin-dependent kinases (CDKs) are core components of the cell cycle machinery that govern the transition between phases during cell cycle progression. Genes involved in cell cycle are frequently mutated in human cancer and deregulated CDK activity represents a hallmark of malignancy. This knowledge provides a rationale for regarding CDKs and their associated molecules as potential targets for new drug development in anticancer research. The present article will review the most relevant CDK inhibitors with emphasis on the newer molecules in clinical development and the biological rationale of this therapeutic approach.


Similar content being viewed by others
References
Harper JW, Adams PD (2001) Cyclin-dependent kinases. Chem Rev 101(8):2511–2526. doi:10.1021/cr0001030
Schwartz GK, Shah MA (2005) Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol 23(36):9408–9421. doi:10.1200/JCO.2005.01.5594
Malumbres M, Barbacid M (2007) Cell cycle kinases in cancer. Curr Opin Genet Dev 17(1):60–65. doi:10.1016/j.gde.2006.12.008
Ortega S, Malumbres M, Barbacid M (2002) Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 1602(1):73–87
Sausville EA (2002) Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med 8(4):S32–S37. doi:10.1016/S1471-4914(02)02308-0
Tibes R, Jimeno A, Von Hoff D. Phase I dose escalation study of the oral multi-CDK inihibitor PHA-848125. Proc Am Soc Clin Oncol 2008: abstr 3531.
Joshi KS, Rathos MJ, Mahajan P, Wagh V, Shenoy S, Bhatia D (2007) P276-00, a novel cyclin-dependent inhibitor induces G1-G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol Cancer Ther 6(3):926–934. doi:10.1158/1535-7163.MCT-06-0614
Tannock IF, Hill RP, Bristow RG (2005) The Basic Science of Oncology. Mc Graw-Hill Professional, New York
Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S (2003) Cyclin E ablation in the mouse. Cell 114(4):431–443. doi:10.1016/S0092-8674(03)00645-7
An HX, Beckmann MW, Reifenberger G, Bender HG, Niederacher D (1999) Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am J Pathol 154(1):113–118
Mendrzyk F, Radlwimmer B, Joos S, Kokocinski F, Benner A, Stange DE (2005) Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J Clin Oncol 23(34):8853–8862. doi:10.1200/JCO.2005.02.8589
Yurakh AO, Ramos D, Calabuig-Farinas S, Lopez-Guerrero JA, Rubio J, Solsona E (2006) Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol 50(3):506–515. discussion 515doi:10.1016/j.eururo.2006.03.027
Tetsu O, McCormick F (2003) Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3(3):233–245. doi:10.1016/S1535-6108(03)00053-9
Collins I, Garrett MD (2005) Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol 5(4):366–373. doi:10.1016/j.coph.2005.04.009
Oelgeschlager T (2002) Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control. J Cell Physiol 190(2):160–169. doi:10.1002/jcp.10058
Blagosklonny MV (2004) Flavopiridol, an inhibitor of transcription: implications, problems and solutions. Cell Cycle 3(12):1537–1542
Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ (1996) Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res 56(13):2973–2978
Melillo G, Sausville EA, Cloud K, Lahusen T, Varesio L, Senderowicz AM (1999) Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res 59(21):5433–5437
Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL (1998) Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood 92(10):3804–3816
Kitada S, Zapata JM, Andreeff M, Reed JC (2000) Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood 96(2):393–397
Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L (1998) Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol 16(9):2986–2999
Thomas JP, Tutsch KD, Cleary JF, Bailey HH, Arzoomanian R, Alberti D (2002) Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol 50(6):465–472. doi:10.1007/s00280-002-0527-2
Aklilu M, Kindler HL, Donehower RC, Mani S, Vokes EE (2003) Phase II study of flavopiridol in patients with advanced colorectal cancer. Ann Oncol 14(8):1270–1273. doi:10.1093/annonc/mdg343
Burdette-Radoux S, Tozer RG, Lohmann RC, Quirt I, Ernst DS, Walsh W (2004) Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs 22(3):315–322. doi:10.1023/B:DRUG.0000026258.02846.1c
Liu G, Gandara DR, Lara PN Jr, Raghavan D, Doroshow JH, Twardowski P (2004) A Phase II trial of flavopiridol (NSC #649890) in patients with previously untreated metastatic androgen-independent prostate cancer. Clin Cancer Res 10(3):924–928. doi:10.1158/1078-0432.CCR-03-0050
Schwartz GK, Ilson D, Saltz L, O'Reilly E, Tong W, Maslak P (2001) Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J Clin Oncol 19(7):1985–1992
Shapiro GI, Supko JG, Patterson A, Lynch C, Lucca J, Zacarola PF (2001) A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clin Cancer Res 7(6):1590–1599
Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B (2007) Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 109(2):399–404. doi:10.1182/blood-2006-05-020735
Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK (2003) Flavopiridol enhances the effect of docetaxel in vitro and in vivo in human gastric cancer cells. Mol Cancer Ther 2(6):549–555
Shah MA, Kortmansky J, Motwani M, Drobnjak M, Gonen M, Yi S (2005) A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clin Cancer Res 11(10):3836–3845. doi:10.1158/1078-0432.CCR-04-2651
Fornier MN, Rathkopf D, Shah M, Patil S, O'Reilly E, Tse AN (2007) Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res 13(19):5841–5846. doi:10.1158/1078-0432.CCR-07-1218
Carvajal RD, Shah MA, Tse R. A phase II study of docetaxel followed by flavopiridol in advanced, gemcitabine-refractory pancreatic cancer. Proc Am Soc Clin Oncol abstr 2008:Abstract 15558.
Bible KC, Lensing JL, Nelson SA, Lee YK, Reid JM, Ames MM (2005) Phase 1 trial of flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic end points. Clin Cancer Res 11(16):5935–5941. doi:10.1158/1078-0432.CCR-04-2566
George S, Kasimis BS, Cogswell J, Schwarzenberger P, Shapiro GI, Fidias P (2008) Phase I study of flavopiridol in combination with Paclitaxel and Carboplatin in patients with non-small-cell lung cancer. Clin Lung Cancer 9(3):160–165. doi:10.3816/CLC.2008.n.024
Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN (2000) The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res 60(8):2108–2112
Monks A, Harris ED, Vaigro-Wolff A, Hose CD, Connelly JW, Sausville EA (2000) UCN-01 enhances the in vitro toxicity of clinical agents in human tumor cell lines. Invest New Drugs 18(2):95–107. doi:10.1023/A:1006313611677
Kortmansky J, Shah MA, Kaubisch A, Weyerbacher A, Yi S, Tong W (2005) Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol 23(9):1875–1884. doi:10.1200/JCO.2005.03.116
Sausville EA, Arbuck SG, Messmann R, Headlee D, Bauer KS, Lush RM (2001) Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol 19(8):2319–2333
Welch S, Hirte HW, Carey MS, Hotte SJ, Tsao MS, Brown S (2007) UCN-01 in combination with topotecan in patients with advanced recurrent ovarian cancer: a study of the Princess Margaret Hospital Phase II consortium. Gynecol Oncol 106(2):305–310. doi:10.1016/j.ygyno.2007.02.018
Lara PN Jr, Mack PC, Synold T, Frankel P, Longmate J, Gumerlock PH (2005) The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res 11(12):4444–4450. doi:10.1158/1078-0432.CCR-04-2602
Meijer L, Raymond E (2003) Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res 36(6):417–425. doi:10.1021/ar0201198
Whittaker SR, Walton MI, Garrett MD, Workman P (2004) The Cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of Cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Res 64(1):262–272. doi:10.1158/0008-5472.CAN-03-0110
MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A (2005) Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res 65(12):5399–5407. doi:10.1158/0008-5472.CAN-05-0233
Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H (2005) Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma. Blood 106(3):1042–1047. doi:10.1182/blood-2005-01-0320
Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C (2007) A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer 96(1):29–37. doi:10.1038/sj.bjc.6603509
Bettayeb K, Sallam H, Ferandin Y, Popowycz F, Fournet G, Hassan M (2008) N-&-N, a new class of cell death-inducing kinase inhibitors derived from the purine roscovitine. Mol Cancer Ther 7(9):2713–2724. doi:10.1158/1535-7163.MCT-08-0080
Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R (2003) Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35(1):25–31. doi:10.1038/ng1232
Muller-Tidow C, Metzger R, Kugler K, Diederichs S, Idos G, Thomas M (2001) Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res 61(2):647–653
Hunt KK, Keyomarsi K (2005) Cyclin E as a prognostic and predictive marker in breast cancer. Semin Cancer Biol 15(4):319–326. doi:10.1016/j.semcancer.2005.04.007
Heath EI, Bible K, Martell RE, Adelman DC, Lorusso PM (2008) A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Invest New Drugs 26(1):59–65. doi:10.1007/s10637-007-9090-3
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3(11):1427–1438
Menu E, Garcia J, Huang X, Di Liberto M, Toogood PL, Chen I (2008) A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res 68(14):5519–5523. doi:10.1158/0008-5472.CAN-07-6404
Joshi KS, Rathos MJ, Joshi RD, Sivakumar M, Mascarenhas M, Kamble S (2007) In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol Cancer Ther 6(3):918–925. doi:10.1158/1535-7163.MCT-06-0613
Hirte HW, Baetz T, Vidal L, Fleming GF, Sugimoto AK, Morgan RJ et al. A phase 1 study of the selective cyclin dependent kinase inhibitor P276-00 in patients with advanced refractory neoplasms. Proc Am Soc Clin Oncol 2007:abstr 14117.
DePinto W, Chu XJ, Yin X, Smith M, Packman K, Goelzer P (2006) In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol Cancer Ther 5(11):2644–2658. doi:10.1158/1535-7163.MCT-06-0355
Diab S, Eckhardt S, Tan A, Frenette G, Gore L, Depinto W et al A phase I study of R547, a novel selective inhibitor of cell cycle and transcriptional cyclin dependent kinases (CDKs). Proc Am Soc Clin Oncol 2007:Abstract 3528.
Camidge DR, Smethurst D, Growcott J, Barrass NC, Foster JR, Febbraro S (2007) A first-in-man phase I tolerability and pharmacokinetic study of the cyclin-dependent kinase-inhibitor AZD5438 in healthy male volunteers. Cancer Chemother Pharmacol 60(3):391–398. doi:10.1007/s00280-006-0371-x
Camidge DR, Pemberton M, Growcott J, Amakye D, Wilson D, Swaisland H (2007) A phase I pharmacodynamic study of the effects of the cyclin-dependent kinase-inhibitor AZD5438 on cell cycle markers within the buccal mucosa, plucked scalp hairs and peripheral blood mononucleocytes of healthy male volunteers. Cancer Chemother Pharmacol 60(4):479–488. doi:10.1007/s00280-006-0387-2
Siemeister G, Luecking U, Wagner C, Detjen K, Mc Coy C, Bosslet K (2006) Molecular and pharmacodynamic characteristics of the novel multi-target tumor growth inhibitor ZK 304709. Biomed Pharmacother 60(6):269–272. doi:10.1016/j.biopha.2006.06.003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz-Padilla, I., Siu, L.L. & Duran, I. Cyclin-dependent kinase inhibitors as potential targeted anticancer agents. Invest New Drugs 27, 586–594 (2009). https://doi.org/10.1007/s10637-009-9236-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9236-6