Summary
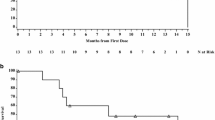
From February, 2001 to September, 2002, the Southwest Oncology Group (SWOG) accrued 65 patients with advanced gastric adenocarcinoma to a phase II trial of weekly 5-FU, leucovorin, and the orally-administered uridine analog PN401. Of these 65 patients, 57 were assessable for survival and toxicity, which were the endpoints for the study. Treatment consisted of the administration of 1200 mg/m2 of 5-FU, 500 mg/m2 of leucovorin, and 6 grams of PN401 every 8 h, beginning 8 h after the completion of the 5-FU infusion, and continuing for a total of 8 doses (48 grams) during each weekly chemotherapy session. Therapy was delivered for six weeks out of every 8-week treatment cycle. The gastrointestinal toxicity of this regimen was mild with 2 patients experiencing grade 3 stomatitis, and 6 patients having grade 3 diarrhea; and the hematologic toxicity was acceptable with 6 of 57 patients found to have had grade 3 or 4 leukopenia, and 14 of 57 patients experiencing grade 3 or 4 neutropenia. There were two deaths judged possibly related to treatment; one in a patient who experienced a variety of Grade 2 gastrointestinal toxicities and died at home with an unknown cause of death; and a second patient who also died at home, and for whom treatment-related sepsis could not be ruled out. The overall median survival was 7.2 months. The ability to safely deliver twice the usual dose of 5-FU with leucovorin on a weekly schedule suggests that oral uridine analog supplementation with PN401 may enhance the therapeutic index of the fluoropyrimidines.
Similar content being viewed by others
References
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ (2003) Cancer statistics, 2003. CA Cancer J Clin 53:5–26
Macdonald JS (2002) Advances in the therapy of gastric cancer. Gastric Cancer 5(Suppl 1):35–40
Berenberg JL, Tangen C, Macdonald JS, Hutchins LF, Natale RB, Oishi N, Guy JT, Fleming TR (1995) Phase II study of 5-fluorouracil and folinic acid in the treatment of patients with advanced gastric cancer. A Southwest Oncology Group study. Cancer 76:715–719
Blanke CD, Haller DG, Benson AB, Rothenberg ML, Berlin J, Mori M, Hsieh YC, Miller LL (2001) A phase II study of irinotecan with 5-fluorouracil and leucovorin in patients with previously untreated gastric adenocarcinoma. Ann Oncol 12:1575–1580
Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, Kim NK (2003) Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol 14:383–387
Kohne CH, Catane R, Klein B, Ducreux M, Thuss-Patience P, Niederle N, Gips M, Preusser P, Knuth A, Clemens M, Bugat R, Figer I, Shani A, Fages B, Di Betta D, Jacques C, Wilke HJ (2003) Irinotecan is active in chemonaive patients with metastatic gastric cancer: A phase II multicentric trial. Br J Cancer 89:997–1001
Haller DG, Misset JL (2002) Docetaxel in advanced gastric cancer. Anticancer Drugs 13:451–460
Tsai JY, Safran H (2003) Status of treatment for advanced gastric carcinoma. Curr Oncol Rep 5:210–218
Gadgeel SM, Shields AF, Heilbrun LK, Labadidi S, Zalupski M, Chaplen R, Philip PA (2003) Phase II study of paclitaxel and carboplatin in patients with advanced gastric cancer. Am J Clin Oncol 26:37–41
Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heinicke A, Meyer J, Meyer HJ, Buente H (1989) Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol 7:1310–1317
Kelsen D, Atiq OT, Saltz L, Niedzwiecki D, Ginn D, Chapman D, Heelan R, Lightdale C, Vinciguerra V, Brennan M (1992) FAMTX versus etoposide, doxorubicin, and cisplatin: A random assignment trial in gastric cancer. J Clin Oncol 10:541–548
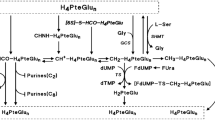
Sobrero AF, Aschele C, Bertino JR (1997) Fluorouracil in colorectal cancer–a tale of two drugs: Implications for biochemical modulation. J Clin Oncol 15:368–381
Rustum YM (1985) Selective modulation of 5-fluorouracil action in patients with colorectal carcinoma. Chemioterapia 4:377–382
Martin DS, Stolfi RL, Sawyer RC, Spiegelman S, Young CW (1982) High-dose 5-fluorouracil with delayed uridine “rescue” in mice. Cancer Res 42:3964–3970
Nord LD, Stolfi RL, Martin DS (1992) Biochemical modulation of 5-fluorouracil with leucovorin or delayed uridine rescue. Correlation of antitumor activity with dosage and FUra incorporation into RNA. Biochem Pharmacol 43:2543–2549
Van Groeningen CJ, Leyva A, Kraal I, Peters GJ, Pinedo HM (1986) Clinical and pharmacokinetic studies of prolonged administration of high-dose uridine intended for rescue from 5-FU toxicity. Cancer Treat Rep 70:745–750
Van Groeningen CJ, Peters GJ, Leyva A, Laurensse E, Pinedo HM (1989) Reversal of 5-fluorouracil-induced myelosuppression by prolonged administration of high-dose uridine. J Natl Cancer Inst 81:157–162
Van Groeningen CJ, Peters GJ, Nadal JC, Laurensse E, Pinedo HM (1991) Clinical and pharmacologic study of orally administered uridine. J Natl Cancer Inst 83:437–441
Kelsen DP, Martin D, O’Neil J, Schwartz G, Saltz L, Sung MT, von Borstel R, Bertino J (1997) Phase I trial of PN401, an oral prodrug of uridine, to prevent toxicity from fluorouracil in patients with advanced cancer. J Clin Oncol 15:1511–1517
Hidalgo M, Villalona-Calero MA, Eckhardt SG, Drengler RL, Rodriguez G, Hammond LA, Diab SG, Weiss G, Garner AM, Campbell E, Davidson K, Louie A, O’Neil JD, von Borstel R, Von Hoff DD, Rowinsky EK (2000) Phase I and pharmacologic study of PN401 and fluorouracil in patients with advanced solid malignancies. J Clin Oncol 18:167–177
Doroshow JH, Carroll M, Leong L, Johnson D, Chow W, Hamasaki V, Margolin K, Morgan RJ, Raschko J, Shibata S, Somlo G, Tetef M, Yen Y, Synold T, Newman E, ter Veer A, Longmate J, von Borstel R, Louie A (1997) Dose escalation trial of 5-FU, leucovorin (LV), and the oral uridine analog PN401 in patients (pts) with metastatic colorectal cancer (cc) who have failed prior 5-FU therapy. Proc Amer Soc Clin Oncol 16:235a
Larsson PA, Carlsson G, Gustavsson B, Graf W, Glimelius B (1996) Different intravenous administration techniques for 5-fluorouracil. Pharmacokinetics and pharmacodynamic effects. Acta Oncol 35:207–212
Novik Y, Ryan LM, Haller DG, Asbury R, Dutcher JP, Schutt A (1999) Phase II protocol for the evaluation of new treatments in patients with advanced gastric carcinoma: Results of ECOG 5282. Med Oncol 16:261–266
Berenberg JL, Tangen C, Macdonald JS, Barlogie B, Laufman LR (1993) VM-26 in gastric cancer. A Southwest Oncology Group study. Invest New Drugs 11:333–334
Benedetti JK, Burris HA III, Balcerzak SP, Macdonald JS (1997) Phase II trial of topotecan in advanced gastric cancer: A Southwest Oncology Group study. Invest New Drugs 15:261–264
Williamson SK, Tangen CM, Maddox AM, Spiridonidis CH, Macdonald JS (1995) Phase II evaluation of low-dose continuous 5-fluorouracil and weekly cisplatin in advanced adenocarcinoma of the stomach. A Southwest Oncology Group study. Am J Clin Oncol 18:484–487
Arbuck SG, Douglass HO Jr, Trave F, Milliron S, Baroni M, Nava H, Emrich LJ, Rustum YM (1987) A phase II trial of 5-fluorouracil and high-dose intravenous leucovorin in gastric carcinoma. J Clin Oncol 5:1150–1156
Collins JM, Dedrick RL, King FG, Speyer JL, Myers CE (1980) Nonlinear pharmacokinetic models for 5-fluorouracil in man: Intravenous and intraperitoneal routes. Clin Pharmacol Ther 28:235–246
Madajewicz S, Petrelli N, Rustum YM, Campbell J, Herrera L, Mittelman A, Perry A, Creaven PJ (1984) Phase I–II trial of high-dose calcium leucovorin and 5-fluorouracil in advanced colorectal cancer. Cancer Res 44:4667–4669
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doroshow, J.H., McCoy, S., Macdonald, J.S. et al. Phase II trial of PN401, 5-FU, and leucovorin in unresectable or metastatic adenocarcinoma of the stomach: A Southwest Oncology Group study. Invest New Drugs 24, 537–542 (2006). https://doi.org/10.1007/s10637-006-9244-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-9244-8