Summary
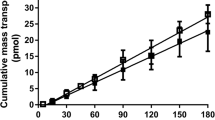
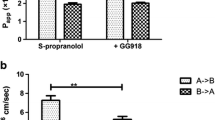
Fenretinide is a synthetic retinoid with chemotherapeutic activity against various malignancies. Upon oral administration to animals, fenretinide was found to be incompletely absorbed and excreted primarily in feces. The purpose of this study was to determine the possible reasons for poor oral absorption of fenretinide using Caco-2 cell monolayers. To achieve this purpose, a solid dispersion of fenretinide with Povidone K25 was used. The apparent permeability coefficient (P app) of fenretinide across Caco-2 monolayers in the presence of bovine serum albumin (BSA) in the receiver was determined. Apical to basolateral (AP-BL) and basolateral to apical (BL-AP) flux studies were performed to determine the role of an efflux mechanism. In the presence of 4% BSA in the receiver, the P app was found to be (8.8 ± 0.5) × 10−8 cm/sec. The AP-BL flux increased linearly with an increase in fenretinide concentration (125–640 μM) in the presence of 4% BSA in the receiver. Efflux and paracellular pathways played an insignificant role in the permeability of fenretinide. A significant amount of drug, approximately 13–15% of the initial amount accumulated in the cell membrane. The amount of fenretinide in the donor decreased by 16% over a 3 h period. However, only 0.12% of the initial amount was found in the receiver. Also, the P app increased with an increase in plasma protein concentration in the receiver. On the basis of these results, the poor permeability of fenretinide can be attributed to its accumulation in the lipophilic cell membrane and poor partitioning into the receiver medium.






Similar content being viewed by others
References
Pollard M, Luckert PH, Sporn MB (1991) Prevention of primary prostate cancer in Lobund-Wistar rats by N-(4-hydroxyphenyl) retinamide. Cancer Res 51:3610–3611
Formelli F, Barua AB, Olson JA (1996) Bioactivities of N-(4-hydroxyphenyl) retinamide and retinoyl beta-glucuronide. Faseb J 10:1014–1024
Lieberman R, Crowell JA, Hawk ET, Boone CW, Sigman CC, Kelloff GJ (1998) Development of new cancer chemoprevention agents: role of pharmacokinetic/pharmacodynamic and intermediate endpoint biomarker monitoring. Clin Chem 44:420–427
Veronesi U, De Palo G, Costa A, Formelli F, Decensi A (1996) Chemoprevention of breast cancer with fenretinide. IARC Sci Publ 87–94
Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L, Decensi A, Camerini T, Del Turco MR, Di Mauro MG, Muraca MG, Del Vecchio M, Pinto C, D’Aiuto G, Boni C, Campa T, Magni A, Miceli R, Perloff M, Malone WF, Sporn MB (1999) Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst 91:1847–1856
Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, Di Mauro MG, Costa A, Marubini E, Sporn MB, De Palo G (2006) Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol 17:1065–1071
Fontana JA, Rishi AK (2002) Classical and novel retinoids: their targets in cancer therapy. Leukemia 16:463–472
Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ (2003) Retinoid therapy of high-risk neuroblastoma. Cancer Lett 197:185–192
Swanson BN, Zaharevitz DW, Sporn MB (1980) Pharmacokinetics of N-(4-Hydroxyphenyl)-all-trans-retinamide in rats. Drug Metab Dispos 8:168–172
Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I (2004) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445
Law D, Krill SL, Schmitt EA, Fort JJ, Qiu Y, Wang W, Porter WR (2001) Physicochemical considerations in the preparation of amorphous ritonavir-poly(ethylene glycol) 8000 solid dispersions. J Pharm Sci 90:1015–1025
Lennernas H (1998) Human intestinal permeability. J Pharm Sci 87:403–410
Ooie T, Terasaki T, Suzuki H, Sugiyama Y (1997) Quantitative brain microdialysis study on the mechanism of quinolones distribution in the central nervous system. Drug Metab Dispos 25:784–789
Fisher JM, Wrighton SA, Calamia JC, Shen DD, Kunze KL, Thummel KE (1999) Midazolam metabolism by modified Caco-2 monolayers: effects of extracellular protein binding. J Pharmacol Exp Ther 289:1143–1150
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci 10:195–204
Gan LS, Hsyu PH, Pritchard JF, Thakker D (1993) Mechanism of intestinal absorption of ranitidine and ondansetron: transport across Caco-2 cell monolayers. Pharm Res 10:1722–1725
Tantishaiyakul V, Wiwattanawongsa K, Pinsuwan S, Kasiwong S, Phadoongsombut N, Kaewnopparat S, Kaewnopparat N, Rojanasakul Y (2002) Characterization of mefenamic acid-guaiacol ester: stability and transport across Caco-2 cell monolayers. Pharm Res 19:1013–1018
Krishna G, Chen K, Lin C, Nomeir AA (2001) Permeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2. Int J Pharm 222:77–89
Saha P, Kou JH (2002) Effect of bovine serum albumin on drug permeability estimation across Caco-2 monolayers. Eur J Pharm Biopharm 54:319–324
Aungst BJ, Nguyen NH, Bulgarelli JP, Oates-Lenz K (2000) The influence of donor and reservoir additives on Caco-2 permeability and secretory transport of HIV protease inhibitors and other lipophilic compounds. Pharm Res 17:1175–1180
Higaki K, Asai M, Suyama T, Nakayama K, Ogawara K, Kimura T (2002) Estimation of intradermal disposition kinetics of drugs: II. Factors determining penetration of drugs from viable skin to muscular layer. Int J Pharm 239:129–141
He YL, Tsujimoto S, Tanimoto M, Okutani R, Murakawa K, Tashiro C (2000) Effects of protein binding on the placental transfer of propofol in the human dually perfused cotyledon in-vitro. Br J Anaesth 85:281–286
Pal D, Udata C, Mitra AK (2000) Transport of cosalane-a highly lipophilic novel anti-HIV agent-across caco-2 cell monolayers. J Pharm Sci 89:826–833
Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, Veronesi U (1993) Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol 11:2036–2042
Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM (2000) Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 21:1271–1279
Zanotti G, Marcello M, Malpeli G, Folli C, Sartori G, Berni R (1994) Crystallographic studies on complexes between retinoids and plasma retinol-binding protein. J Biol Chem 269:29613–29620
de Pee S, Dary O (2002) Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 132:2895S–2901S
Sawada GA, Ho NF, Williams LR, Barsuhn CL, Raub TJ (1994) Transcellular permeability of chlorpromazine demonstrating the roles of protein binding and membrane partitioning. Pharm Res 11:665–673
Wils P, Warnery A, Phung-Ba V, Legrain S, Scherman D (1994) High lipophilicity decreases drug transport across intestinal epithelial cells. J Pharmacol Exp Ther 269:654–658
Rubas W, Cromwell M (1997) The effect of chemical modifications on octanol/water partition (log D) and permeabilities across Caco-2 monolayers. Adv Drug Deliv Rev 23:157–162
Sawada GA, Barsuhn CL, Lutzke BS, Houghton ME, Padbury GE, Ho NF, Raub TJ (1999) Increased lipophilicity and subsequent cell partitioning decrease passive transcellular diffusion of novel, highly lipophilic antioxidants. J Pharmacol Exp Ther 288:1317–1326
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokate, A., Li, X. & Jasti, B. Transport of a novel anti-cancer agent, fenretinide across Caco-2 monolayers. Invest New Drugs 25, 197–203 (2007). https://doi.org/10.1007/s10637-006-9026-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-9026-3