Summary
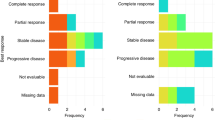
Purpose: Protein Kinase C (PKC), involved in transmembrane signaling of cell surface receptors, promotes carcinogenesis and tumor progression. Bryostatin-1 competes with PKC for phorbol esters (tumor promoters), thus inhibiting tumor progression. Bryostatin-1 also increases cytotoxicity of paclitaxel in a sequential fashion. We studied sequential paclitaxel and bryostatin-1 in patients with untreated, advanced gastric adenocarcinoma. Methods: Patients with histologic proof of gastric or gastroesophageal junction adenocarcinoma with advanced, measurable cancers were eligible. Patients were required to have near normal organ function and ECOG performance status of 0 or 1. All patients gave an informed consent. Patients received paclitaxel 80 mg/m2 in 2 h intravenously on day 1 and bryostatin-1 40 mcg/m2 in 1 h intravenously on day 2 each week for 3 consecutive weeks out of 4. Primary objective was to assess the objective response rate. Results: In a multi-center setting, 37 patients were enrolled and 35 were assessable for response. A confirmed partial response rate was 29%. The median time-to-progression was 4.25 months and the median survival time was 8 months. Grade 3 cumulative myalgias occurred in 55% of patients. Twelve patients discontinued therapy due to myalgias, including 6 patients who had not progressed after achieving a partial response. Other toxic effects were uncommon. Conclusions: Sequential paclitaxel plus bryostatin-1 resulted in a superior response rate than would be expected of paclitaxel alone in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Further development of this combination is warranted once an effective method to ameliorate or prevent myalgias can be established.
Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–08
Spitaler M, Cantrell DA (2004) Protein kinase C and beyond. Nat Immunol 5:785–90
Blumberg PM (1988) Protein kinase C as the receptor for the phorbo ester tumor promoters: Sixth Rhoades memorial award lecture. Cancer Res 48:1–
Hofmann J (2004) Protein kinase C isoenzymes as potential targets for anticancer therapy. Curr Cancer Drug Targets 4:125–46
Pettit GR, Herald CL, Doubet DL, et al (1982) Isolation and structure of bryostatin-1. J Am Chem Soc 104:6846–848
Gschwendt M, Furstenberger G, Rose-John S, et al (1988) Bryostatin-1, an activator of protein kinase C, mimics as well as inhibits biological effects of the phorbol ester TPA in vivo and in vitro. Carcinogenesis 9:555–62
Hennings H, Blumberg PM, Pettit GR, et al (1987) Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis 8:1343–346
Schuchter LM, Esa AH, May S, et al (1991) Successful treatment of murine melanoma with bryostatin-1. Cancer Res 51:682–87
Hornung RL, Pearson JW, Beckwith M, et al (1992) Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res 52:101–07
Jones RJ, Sharkis SJ, Miller CB, et al (1990) Bryostatin-1, a unique biologic response modifier: Anti-leukemic activity in vitro. Blood 75:1319–323
Mohammad RM, Diwakaran H, Maki A, et al (1995) Bryostatin-1 induces apoptosis and augments inhibitory effects of vincristine in human diffuse large cell lymphoma. Leuk Res 19:667–73
Grant S, Jarvis WD, Swerdlow PS, et al (1992) Potentiation of the activity of 1-D-arabinofuranosylcytosine by the protein kinase C activator bryostatin-1 in HL-60 cells: association with enhanced fragmentation of mature DNA. Cancer Res 52:6270–278
Koutcher JA, Motwani M, Zakian KL, et al (2000) The in vitro effect of bryostatin-1 on paclitaxel-induced tumor growth, mitotic entry, and blood flow. Clin Cancer Res 6:1498–507
Ajani JA, Fairweather J, Dumas P, et al (1998) Phase II study of Taxol in patients with advacd gastric carcinoma. Cancer J Sci Am 4:269–74
Asiedu C, Biggs J, Lilly M, Kraft AS (1995) Inhibition of lleukemic cell growth by the protein kinase C activator bryostatin-1 correlates with the dephosphorylation of cyclin dependent kinase 2. Cancer Res 55:3716–720
Kaubisch A, Kelsen DP, Saltz L, Kemeny N, O’Reilly E, Ilson D, Endres S, Barazzuol J, Schwartz GK (1999) A phase I trial of weekly sequential bryostatin-1 (BRYO) and paclitaxel in patients with advanced solid tumors. Proc Amer Soc Clin Onc 18:166a
Author information
Authors and Affiliations
Additional information
Supported in part by the NCI phase II contract no. N01-CM-1703 and CCOP contract no. 5 U10 CA 045809-15
Rights and permissions
About this article
Cite this article
Ajani, J.A., Jiang, Y., Faust, J. et al. A multi-center phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Invest New Drugs 24, 353–357 (2006). https://doi.org/10.1007/s10637-006-6452-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-6452-1