Summary
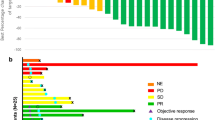
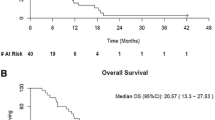
Purpose: Fenretinide, a synthetic form of retinoid, induced apoptosis even in chemotherapy resistant cell lines. A phase II study was hence conducted to evaluate toxicity and efficacy of fenretinide in metastatic renal cancer. Methods: Eligibility included unresectable or metastatic renal cell carcinoma (RCC), adequate organ function and Zubrod performance status ≦2. Prior immunotherapy and a maximum of one prior chemotherapy regimen were allowed. Fenretinide was administered at a dose of 900 mg/m2 twice daily orally for 7 days in a 21-day cycle. Toxicity was assessed at the start of each cycle, and response every 2 cycles. Results: Nineteen eligible patients enrolled of which fifteen had visceral/bone metastases. Seventeen patients had prior nephrectomy and 11 had prior immunotherapy. 76 cycles of therapy were delivered. Therapy was very well tolerated with few severe toxicities consisting of thrombosis in 1 individual and grade 3 fatigue, nausea and diarrhea in 1 patient. 5 patients had grade 2 nyctalopia and 3 patients had transient grade 2 visual toxicity. No objective responses were noted. Stable disease was seen in seven of nineteen cases (37%, 90% C.I. 0.21–0.59). Median time to progression was 1.5 months and median duration of stable disease was 5.8 months (90% C.I. 3.0–8.4). Median survival was 10 months. Tumor fenretinide levels were obtained in three patients and were in the lower end of the therapeutic range. Conclusion: Fenretinide was well tolerated but demonstrated minimal activity that was consistent with results of intratumoral drug measurements. Strategies are needed that will increase systemic and tumor levels of fenretinide.
Similar content being viewed by others
References
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM et al.: All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 337:1021–1028, 1997
Camacho LH: Clinical applications of retinoids in cancer medicine. J Biol Regul Homeost Agents. Jan-Mar 17:98–114, 2003
Taguchi I, Hara I, Gohji K, Arakawa S, Kamidono S: Synergistic anti-tumor effect of 13-cRA and IFN-alpha/beta in mouse renal cell carcinoma. Int J Oncol 13:145–149, 1998
Motzer RJ, Murphy BA, Bacik J, Schwartz LH, Nanus DM, Mariani T, Loehrer P, Wilding G, Fairclough DL, Cella D et al.: Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 18:2972–2980, 2000
Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Grignani F, Formelli F, Menard S, Costa A, Veronesi U et al.: N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res 53:6036–6041, 1993
Tosetti F, Vene R, Arena G, Morini M, Minghelli S, Noonan DM, Albini A: N-(4-hydroxyphenyl)retinamide inhibits retinoblastoma growth through reactive oxygen species-mediated cell death. Mol Pharmacol 63:565–573, 2003
Costa A, Malone W, Perloff M, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M, Andreoli C, Del Vecchio M et al.: Tolerability of the synthetic retinoid Fenretinide (HPR). Eur J Cancer Clin Oncol 25:805–808, 1989
Torrisi R, Decensi A, Formelli F, Camerini T, De Palo G: Chemoprevention of breast cancer with fenretinide. Drugs 61:909–918, 2001
Chiesa F, Tradati N, Marazza M, Rossi N, Boracchi P, Mariani L, Fornelli F, giardini R, Costa A, DePalo G et al.: Fenretinide (4-HPR) in chemoprevention of oral leukoplakia. J Cell Biochem Suppl 17F:255–261, 1993
Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M: Plasma retinol level reduction by the synthetic retinoid fenretinide: A one year follow-up study of breast cancer patients. Cancer Res 49:6149–1652, 1989
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein, L, Verweij, J, Van Glabbeke, M, van Oosterom, AT, Christian MC et al.: New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216, 2000
Fleming TR: One-sample multiple testing procedure for Phase II clinical trials. Biometrics 38:143–151, 1982
Casella G: Refining binomial confidence intervals. Canadian J Statistics 14: 113–129, 1987
Mehta C, Patel N: StatXact 5:Statistical Software for Exact Nonparametric Inference, User Manual. Cytel Software Corporation, Cambridge, MA 1999, pp. 429–433
Lee ET: Statistical Methods for Survival Data Analysis, 2nd Edition Wiley & Sons, Inc, New York, 1992, pp. 77–78
Motzer RJ, Schwartz L, Law TM, Murphy BA, Hoffman AD, Albino AP, Vlamis V, Nanus DM: Interferon alfa-2a and 13-cis-retinoic acid in renal cell carcinoma: Antitumor activity in a phase II trial and interactions in vitro. J Clin Oncol 13:1950–1907, 1995
Nanus DM, Geng Y, Shen R, Lai HK, Pfeffer SR and Pfeffer LM Interaction of retinoic acid, interferon in renal cancer cell lines. J Interferon Cytokine Res 20:787–794, 2000
Delia D, Aiello A, Formelli F, Fontanella E, Costa A, Miyashita T, Reed JC, Pierotti MA: Regulation of apoptosis induced by the retinoid N-(4-hydroxyphenyl) retinamide and effect of deregulated bcl-2. Blood 85:359–367, 1995
Sun SY, Li W, Yue P, Lippman SM, Hong WK, Lotan R: Mediation of N-(4-hydoxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res 59:2493–2498, 1999
Dipietrantonio A, Hsieh TC, Wu JM: Differential effects of retinoic acid (RA) and N-(4-hydroxyphenyl) retinamide (4-HPR) on cell growth, induction of differentiation, and changes in p34cdc2, Bcl-2 and actin expression in the human promyelocytic HL-60 leukemic cells. Biochem Biophys Res Commun 224:837–842, 1996
Fontana JA, Rishi AK: Classical and novel retinoids: Their targets in cancer therapy. Leukemia 16:463–472, 2002
Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, Costa A, Veronesi U: Safety of the synthetic retinoid fenretinide: Long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol 19:1664–1670, 2001
Garaventa A, Luksch R, Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, Bernardi BD, Bellani FF, Formelli F: Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res 9:2032–2039, 2003
Kalemkerian GP, Slusher R, Ramalingam S, Gadgeel S, Mabry M: Growth inhibition and induction of apoptosis by fenretinide in small-cell lung cancer cell lines. J Natl Cancer Inst 87:1674–1680, 1995
Marth C, Bock G, Daxenbichler G: Effect of 4-hydroxyphenylretinamide and retinoic acid on proliferation and cell cycle of cultured human breast cancer cells. J Natl Cancer Inst 75:871–875, 1985
Oridate N, Lotan D, Mitchell MF, Hong WK, Lotan R: Inhibition of proliferation and induction of apoptosis in cervical carcinoma cells by retinoids: Implications for chemoprevention. J Cell Biochem Suppl 23:80–86, 1995
Mariotti A, Marcora E, Bunone G, Costa A, Veronesi U, Pierotti MA, Della Valle G: N-(4-hydroxyphenyl)retinamide: A potent inducer of apoptosis in human neuroblastoma cells. J Natl Cancer Inst 86:1245–1247, 1994
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by NCI Cancer Center Support Grant CA-22453.
Rights and permissions
About this article
Cite this article
Vaishampayan, U., Heilbrun, L.K., Parchment, R.E. et al. Phase II trial of fenretinide in advanced renal carcinoma. Invest New Drugs 23, 179–185 (2005). https://doi.org/10.1007/s10637-005-5864-7
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-5864-7