Abstract
Purpose
Bevacizumab or temsirolimus regimens have clinical activity in the first-line treatment of advanced renal cell carcinoma (RCC). This phase I/II trial was conducted to determine the safety of combining both agents and its efficacy in RCC patients who progressed on at least one prior anti-VEGF receptor tyrosine kinase inhibitor (RTKI) agent.
Methods
In the phase I portion, eligible patients were treated with temsirolimus (25 mg IV weekly) and escalating doses of IV bevacizumab (level 1 = 5 mg/kg; level 2 = 10 mg/kg) every other week. The primary endpoint for the phase II portion (RTKI resistant patients) was the 6-month progression-free rate. Secondary endpoints were response rate, toxicity evaluation, and PFS and OS.
Results
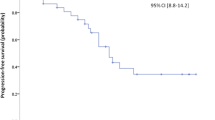
Maximum tolerated dose was not reached at the maximum dose administered in 12 phase I patients. Forty evaluable patients were treated with the phase II recommended dose (temsirolimus 25 mg IV weekly and bevacizumab 10 mg/kg IV every 2 weeks). The 6-month progression-free rate was 40 % (16/40 pts). Median PFS was 5.9 (4–7.8) months, and median OS was 20.6 (11.5–23.7) months. Partial response, stable disease, and progressive disease were seen in 23, 63, and 14 % of patients, respectively. Most common grade 3–4 AEs included fatigue (17.8 %), hypertriglyceridemia (11.1 %), stomatitis (8.9 %), proteinuria (8.9 %), abdominal pain (6.7 %), and anemia (6.7 %). Baseline levels of serum sFLT-1 and VEGF-A were inversely correlated with PFS and OS, respectively.
Conclusions
Temsirolimus and bevacizumab is a feasible combination in patients with advanced RCC previously exposed to oral anti-VEGF agents. The safety and efficacy results warrant further confirmatory studies in this patient population.


Similar content being viewed by others
References
Jonasch E, Futreal PA, Davis IJ, Bailey ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J, Zurita AJ, Rini BI, Sharma P, Atkins MB, Walker CL, Rathmell WK (2012) State of the science: an update on renal cell carcinoma. Mol Cancer Res 10(7):859–880. doi:10.1158/1541-7786.MCR-12-0117
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281. doi:10.1056/NEJMoa066838
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–731. doi:10.1056/NEJMoa1303989
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2010) Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 28(13):2137–2143. doi:10.1200/JCO.2009.26.5561
Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, Wood L, Wang Y, Kollmannsberger C, North S, Donskov F, Rini BI, Choueiri TK (2012) Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol 23(6):1549–1555. doi:10.1093/annonc/mdr533
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124. doi:10.1056/NEJMoa065044
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6):1061–1068. doi:10.1200/JCO.2009.23.9764
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116(18):4256–4265. doi:10.1002/cncr.25219
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
Boss DS, Glen H, Beijnen JH, Keesen M, Morrison R, Tait B, Copalu W, Mazur A, Wanders J, O’Brien JP, Schellens JH, Evans TR (2012) A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer 106(10):1598–1604. doi:10.1038/bjc.2012.154
Vaishampayan U (2013) Cabozantinib as a novel therapy for renal cell carcinoma. Curr Oncol Rep 15(2):76–82. doi:10.1007/s11912-012-0289-x
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. doi:10.1056/NEJMoa1200690
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. doi:10.1038/nm.2883
Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA (2007) Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30(8):825–830. doi:10.1097/CJI.0b013e318156e47e
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22(20):7004–7014
Land SC, Tee AR (2007) Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282(28):20534–20543. doi:10.1074/jbc.M611782200
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Merchan JR, Chan B, Kale S, Schnipper LE, Sukhatme VP (2003) In vitro and in vivo induction of antiangiogenic activity by plasminogen activators and captopril. J Natl Cancer Inst 95(5):388–399
Merchan JR, Jayaram DR, Supko JG, He X, Bubley GJ, Sukhatme VP (2005) Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: potentiation by Cox-2 inhibition. Int J Cancer 113(3):490–498. doi:10.1002/ijc.20595
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Duffy DE, Santner TJ (1987) Confidence intervals for a binomial parameter based on multistage tests. Biometrics:81–93
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27(34):5794–5799. doi:10.1200/JCO.2008.21.4809
Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, Escudier B (2013) Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. doi:10.1200/JCO.2013.50.5305
Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, Blanc E, Ferlay C, Geoffrois L, Rolland F, Legouffe E, Sevin E, Laguerre B, Escudier B (2011) Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 12(7):673–680. doi:10.1016/S1470-2045(11)70124-3
Hainsworth JD, Spigel DR, Burris HA 3rd, Waterhouse D, Clark BL, Whorf R (2010) Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol 28(13):2131–2136. doi:10.1200/JCO.2009.26.3152
Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, Chen C, Rosbrook B, Kim S, Rini BI (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14(6):552–562. doi:10.1016/S1470-2045(13)70093-7
Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, Ravaud A, Golding S, Jethwa S, Sneller V (2010) Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 28(13):2144–2150. doi:10.1200/JCO.2009.26.7849
Ravaud A, Schmidinger M (2013) Clinical biomarkers of response in advanced renal cell carcinoma. Ann Oncol. doi:10.1093/annonc/mdt288
Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31(17):2205–2218. doi:10.1200/JCO.2012.46.3653
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig 111(5):649–658. doi:10.1172/JCI17189
Acknowledgments
We would like to thank Dr Krisztina Kovacs (University of Miami) for technical assistance on the biomarker studies. This work was supported by P2C contract N01-CM62205; Commonwealth Foundation (to CE), and CA 119545-02 (to JRM).
Conflict of interest
P.J.F. is on the speakers’ bureau for Genentech and Novartis. All other authors disclosed no conflicts.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
“Informed consent was obtained from all individual participants included in the study.”
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Merchan, J.R., Qin, R., Pitot, H. et al. Safety and activity of temsirolimus and bevacizumab in patients with advanced renal cell carcinoma previously treated with tyrosine kinase inhibitors: a phase 2 consortium study. Cancer Chemother Pharmacol 75, 485–493 (2015). https://doi.org/10.1007/s00280-014-2668-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2668-5