Summary
Background. Pancreatic cancer is amongst the most chemoresistant malignancies. Expression of the cyclooxygenase-2 (COX-2) enzyme plays a major role in tumor progression and resistance to therapy. A Phase II study was undertaken to determine the effect of gemcitabine by fixed-dose rate infusion (FDR), cisplatin and the COX-2 inhibitor, celecoxib, on the 6-month survival rate in patients with metastatic pancreatic cancer.
Methods. The eligibility criteria included a pathologically or cytologically confirmed diagnosis of adenocarcinoma of the pancreas. No prior gemcitabine therapy was allowed. Patients received a combination of gemcitabine 1000 mg/m2 over 100 minutes, cisplatin 35 mg/m2 I.V. on days 1 and 8, and celecoxib continuously at a daily dose of 800 mg. Cycles were repeated every 21 days.
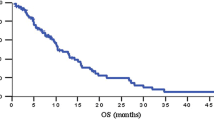
Results. Twenty-two patients with metastatic pancreas cancer were enrolled (median age, 59.5 years; M:F, 13:9). The median number of cycles was 2 per patient. The median survival time was 5.8 months (90% CI, 3.6–7.6 months). The probability of survival at 6 months was 46% (90% CI, 27–62%). The major toxicity was neutropenia with grade 3 or 4 toxicities seen in 65% of patients.
Conclusions. The addition of celecoxib to gemcitabine (by FDR) and cisplatin did not appear to increase activity of the chemotherapy doublet in patients with advanced pancreatic cancer. Celecoxib alone may not be sufficient to sensitize pancreatic cancer to the effects of conventional cytotoxic therapy.
Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ, American Cancer Society: Cancer statistics, 2004. CA Cancer J Clin 54(1): 8–29, 2004
El-Rayes BF, Shields AF, Vaitkevicius V, Philip PA: Developments in the systemic therapy of pancreatic cancer. Cancer Invest 21(1): 73–86, 2003
Funk CD: Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294(5548): 1871–1875, 2001
Ding XZ, Hennig R, Adrian TE: Lipoxygenase and cyclooxygenase metabolism: New insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer 2(1): 10, 2003
Merati K, said Siadaty M, Andea A, Sarkar F, Ben-Josef E, Mohammad R, Philip P, Shields AF, Vaitkevicius V, Grignon DJ, Adsay NV: Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol 24(5): 447–452, 2001
Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ: Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 21(2): 139–146, 2000
Sheng H, Shao J, Washington MK, DuBois RN: Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem 276(21): 18075–18081, 2001
Gately S: The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev 19(1–2): 19–27, 2000
Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC: Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res 63(3): 728–734, 2003
Sheng H, Shao J, Dubois RN: K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res 61(6): 2670–2675, 2001
Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: Growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res 59(17): 4356–4362, 1999
Yip-Schneider MT, Sweeney CJ, Jung SH, Crowell PL, Marshall MS: Cell cycle effects of nonsteroidal anti-inflammatory drugs and enhanced growth inhibition in combination with gemcitabine in pancreatic carcinoma cells. J Pharmacol Exp Ther 298(3): 976–985, 2001
El-Rayes BF, Ali S, Sarkar FH, Philip PA: Cyclooxygenase-2-dependent and -independent effects of celecoxib in pancreatic cancer cell lines. Mol Cancer Ther 3(11): 1421–1426, 2004
Whelton A, Fort JG, Puma JA, Normandin D, Bello AE, Verburg KM: Cyclooxygenase-2–specific inhibitors and cardiorenal function: A randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther 8(2): 85–95, 2001
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS: Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. Jama 284(10): 1247–1255, 2000
Burris H, Storniolo AM: Assessing clinical benefit in the treatment of pancreas cancer: Gemcitabine compared to 5-fluorouracil. Eur J Cancer 33(Suppl 1): S18–22, 1997
Philip PA, Zalupski MM, Vaitkevicius VK, Arlauskas P, Chaplen R, Heilbrun LK, Adsay V, Weaver D, Shields AF: Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer 92(3): 569–577, 2001
Heinemann V, Wilke H, Mergenthaler HG, et al: Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol 11(11): 1399–1403, 2000
Colucci G, Giuliani F, Gebbia V, Bilietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M: Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: A prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 94(4): 902–910, 2002
Heinemann V, Quietzsch D, Gieseler F, Gonnermann H. A phase III study comparin gemcitabine and cisplatin to gemcitaine alone in advanced pancreatic cancer. Paper presented at: American Society Clinical Oncology, 2003; Chicago, IL.
Touroutoglou N, Gravel D, Raber MN, Plunkett W, Abbruzzese JL. Clinical results of a pharmacodynamically-based strategy for higher dosing of gemcitabine in patients with solid tumors. Ann Oncol 9(9): 1003–1008, 1998
Tempero M, Plunkett W, Ruiz Van Haperen V, et al: Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21(18): 3402–3408, 2003
Simon R. Optimal two-stage designs for Phase II clinical trials. Controlled Clinical Trials 10: 1–10, 1989
Casella G. Refining binomial confidence intervals. Canadian J. Statistics 14: 113–129, 1987
Mehta C, Patel N: StatXact 5: Statistical software for exact nonparametric inference, user manual: Cytel Software Corporation; 1999.
Lee E: Statistical Methods for Survival Data Analysis, 2nd ed. Wiley & Sons, Inc., 1992.
Altorki NK, Keresztes RS, Port JL, et al: Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol 21(14): 2645–2650, 2003
Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fijimura T, Su LK, Levin B: The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 342(26): 1946–1952, 2000
Ebil G, Reber H, Okada Y, Rix T, Hines O: The selective COX-2 inhibitor nimesulide stimulates angiogenesis and growth of COX-2 negative pancreatic cancer in vivo. Paper presented at: Gastroinestinal Cancer Symposium; January 22–24, 2004; San Franscisco, Ca.
Smith SE, Burris HA, 3rd, Loehrer PJ, Sr., Sweeny C: Preliminary report of a Phase II trial of gemcitabine combined with celecoxib for advanced pancreatic cancer. Paper presented at: American Society Clinical Oncology; May 31–June 3, 2003; Chicago, Il.
Lipton A, Harvey H, Witters L, Kerr S, Legore K, Campbell C: Phase II trial of gemcitabine+irinotecan+celecoxib in pancreatic cancer. Paper presented at: Gastrointestinal Cancer Symposium; January 22–24, 2004; San Franscisco, CA.
Lorenz M, Slaughter HS, Wescott DM, Carter SI, Schnyder B, Dinchuk JE, Car BD: Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp Hematol 27(10): 1494–1502, 1999
Ko A, Dito E, Schillinger B, Venook AP, Bersgland EK, Allen JN, Tempero MA: A phase II trial of gemcitabine given at fixed dose infusion in combination with cispplatin for metastatic adenocarcinoma of pancreas. Paper presented at: Gastrointestinal Cancers Symposium; January 22–24, 2004; San Franscisco, CA.
Blanke CD, Benson AB III, Dragovich T, Lenz H, Haller D, Tobles C, Buchbinder A: A phase II trial of celecoxib (CX), irinotecan (I), 5-Fluorouracil (5FU), and leucovorin (LCV) in patients (pts) with unresectable or metastatic colorectal cancer (CRC). Paper presented at: ASCO proceedings; May 18–21, 2002; Orlando, FL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Rayes, B.F., Zalupski, M.M., Shields, A.F. et al. A Phase II study of celecoxib, gemcitabine, and cisplatin in advanced pancreatic cancer. Invest New Drugs 23, 583–590 (2005). https://doi.org/10.1007/s10637-005-1028-z
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-1028-z