Abstract
Purpose
To assess the effect of age and test–retest reliability of the intensity response function of the full-field photopic negative response (PhNR) in normal healthy human subjects.
Methods
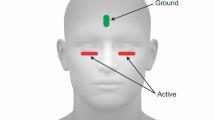
Full-field electroretinograms (ERGs) were recorded from one eye of 45 subjects, and 39 of these subjects were tested on two separate days with a Diagnosys Espion System (Lowell, MA, USA). The visual stimuli consisted of brief (<5 ms) red flashes ranging from 0.00625 to 6.4 phot cd.s/m2, delivered on a constant 7 cd/m2 blue background. PhNR amplitudes were measured at its trough from baseline (BT) and from the preceding b-wave peak (PT), and b-wave amplitude was measured at its peak from the preceding a-wave trough or baseline if the a-wave was not present. The intensity response data of all three ERG measures were fitted with a generalized Naka–Rushton function to derive the saturated amplitude (V max), semisaturation constant (K) and slope (n) parameters. Effect of age on the fit parameters was assessed with linear regression, and test–retest reliability was assessed with the Wilcoxon signed-rank test and Bland–Altman analysis. Holm’s correction was applied to account for multiple comparisons.
Results
V max of BT was significantly smaller than that of PT and b-wave, and the V max of PT and b-wave was not significantly different from each other. The slope parameter n was smallest for BT and the largest for b-wave and the difference between the slopes of all three measures were statistically significant. Small differences observed in the mean values of K for the different measures did not reach statistical significance. The Wilcoxon signed-rank test indicated no significant differences between the two test visits for any of the Naka–Rushton parameters for the three ERG measures, and the Bland–Altman plots indicated that the mean difference between test and retest measurements of the different fit parameters was close to zero and within 6% of the average of the test and retest values of the respective parameters for all three ERG measurements, indicating minimal bias. While the coefficient of reliability (COR, defined as 1.96 times the standard deviation of the test and retest difference) of each fit parameter was more or less comparable across the three ERG measurements, the %COR (COR normalized to the mean test and retest measures) was generally larger for BT compared to both PT and b-wave for each fit parameter. The Naka–Rushton fit parameters did not show statistically significant changes with age for any of the ERG measures when corrections were applied for multiple comparisons. However, the V max of BT demonstrated a weak correlation with age prior to correction for multiple comparisons, and the effect of age on this parameter showed greater significance when the measure was expressed as a ratio of the V max of b-wave from the same subject.
Conclusion
V max of the BT amplitude measure of PhNR at the best was weakly correlated with age. None of the other parameters of the Naka–Rushton fit to the intensity response data of either the PhNR or the b-wave showed any systematic changes with age. The test–retest reliability of the fit parameters for PhNR BT amplitude measurements appears to be lower than those of the PhNR PT and b-wave amplitude measurements.







Similar content being viewed by others
References
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522
Drasdo N, Aldebasi YH, Chiti Z, Mortlock KE, Morgan JE, North RV (2001) The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:1266–1272
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122:341–346
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45:3827–3837
Machida S, Gotoh Y, Tanaka M, Tazawa Y (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 137:938–940
Ueno S, Kondo M, Piao CH, Ikenoya K, Miyake Y, Terasaki H (2006) Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG-assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci 47:3545–3549
Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50:367–373
Chen H, Wu D, Huang S, Yan H (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113:53–59
Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, Terasaki H (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48:820–824
Chen H, Zhang M, Huang S, Wu D (2008) The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol 117:129–135
Shinoda K, Yamada K, Matsumoto CS, Kimoto K, Nakatsuka K (2008) Changes in retinal thickness are correlated with alterations of electroretinogram in eyes with central retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol 246:949–954
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49:2201–2207
Sustar M, Cvenkel B, Brecelj J (2009) The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol 118:1671–1677
Matsumoto CS, Shinoda K, Yamada K, Nakatsuka K (2009) Photopic negative response reflects severity of ocular circulatory damage after central retinal artery occlusion. Ophthalmologica 223:362–369
North RV, Jones AL, Drasdo N, Wild JM, Morgan JE (2010) Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 51:1216–1222
Matsumoto CS, Shinoda K, Nakatsuka K (2011) High correlation of scotopic and photopic electroretinogram components with severity of central retinal artery occlusion. Clin Ophthalmol 5:115–121
Thompson DA, Feather S, Stanescu HC, Freudenthal B, Zdebik AA, Warth R, Ognjanovic M, Hulton SA, Wassmer E, van’t Hoff W, Russell-Eggitt I, Dobbie A, Sheridan E, Kleta R, Bockenhauer D (2011) Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol 589:1681–1689
Horn FK, Gottschalk K, Mardin CY, Pangeni G, Jünemann AG, Kremers J (2011) On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc Ophthalmol 122:53–62
Machida S, Tamada K, Oikawa T, Gotoh Y, Nishimura T, Kaneko M, Kurosaka D (2011) Comparison of photopic negative response of full-field and focal electroretinograms in detecting glaucomatous eyes. J Ophthalmol 2011:pii
Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52:8527–8533
Gowrisankaran S, Anastasakis A, Fishman GA, Alexander KR (2011) Structural and functional measures of inner retinal integrity following visual acuity improvement in a patient with hereditary motor and sensory neuropathy type VI. Ophthalmic Genet 32:188–192
McFarlane M, Wright T, Stephens D, Nilsson J, Westall CA (2012) Blue flash ERG PhNR changes associated with poor long-term glycemic control in adolescents with type 1 diabetes. Invest Ophthalmol Vis Sci 53:741–748
Pangeni G, Lämmer R, Tornow RP, Horn FK, Kremers J (2012) On- and off-response ERGs elicited by sawtooth stimuli in normal subjects and glaucoma patients. Doc Ophthalmol 124:237–248
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53:1315–1323
Kremers J, Jertila M, Link B, Pangeni G, Horn FK (2012) Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol 124:79–90
Niyadurupola N, Luu CD, Nguyen DQ, Geddes K, Tan GX, Wong CC, Tran T, Coote MA, Crowston JG (2013) Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest Ophthalmol Vis Sci 54:1913–1919
Preiser D, Lagrèze WA, Bach M, Poloschek CM (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54:1182–1191
Topčić IG, Šuštar M, Brecelj J, Hawlina M, Jaki Mekjavić P (2014) Morphological and electrophysiological outcome in prospective intravitreal bevacizumab treatment of macular edema secondary to central retinal vein occlusion. Doc Ophthalmol 129:27–38
Machida S, Toba Y, Nishimura T, Ohzeki T, Murai K, Kurosaka D (2014) Comparisons of cone electroretinograms after indocyanine green-, brilliant blue G-, or triamcinolone acetonide-assisted macular hole surgery. Graefes Arch Clin Exp Ophthalmol 252:1423–1433
Moss HE, Park JC, McAnany JJ (2015) The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56:3709–3714
Abed E, Piccardi M, Rizzo D, Chiaretti A, Ambrosio L, Petroni S, Parrilla R, Dickmann A, Riccardi R, Falsini B (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56:2469–2474
Noma H, Mimura T, Kuse M, Yasuda K, Shimura M (2015) Photopic negative response in branch retinal vein occlusion with macular edema. Int Ophthalmol 35:19–26
Morny EK, Margrain TH, Binns AM, Votruba M (2015) Electrophysiological ON and OFF responses in autosomal dominant optic atrophy. Invest Ophthalmol Vis Sci 56:7629–7637
Kirkiewicz M, Lubiński W, Penkala K (2016) Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc Ophthalmol 132:57–65
Binns AM, Mortlock KE, North RV (2011) The relationship between stimulus intensity and response amplitude for the photopic negative response of the flash electroretinogram. Doc Ophthalmol 122:39–52
Arden GB, Carter RM, Hogg CR, Powell DJ, Ernst WJ, Clover GM, Lyness AL, Quinlan MP (1983) A modified ERG technique and the results obtained in X-linked retinitis pigmentosa. Br J Ophthalmol 67:419–430
Massof RW, Wu L, Finkelstein D, Perry C, Starr SJ, Johnson MA (1984) Properties of electroretinographic intensity-response functions in retinitis pigmentosa. Doc Ophthalmol 57:279–296
Birch DG, Herman WK, deFaller JM, Disbrow DT, Birch EE (1987) The relationship between rod perimetric thresholds and full-field rod ERGs in retinitis pigmentosa. Invest Ophthalmol Vis Sci 28:954–965
Johnson MA, Marcus S, Elman MJ, McPhee TJ (1988) Neovascularization in central retinal vein occlusion: electroretinographic findings. Arch Ophthalmol 106:348–352
Peachey NS, Fishman GA, Derlacki DJ, Alexander KR (1988) Rod and cone dysfunction in carriers of X-linked retinitis pigmentosa. Ophthalmology 95:677–685
Breton ME, Quinn GE, Keene SS, Dahmen JC, Brucker AJ (1989) Electroretinogram parameters at presentation as predictors of rubeosis in central retinal vein occlusion patients. Ophthalmology 96:1343–1352
Birch DG, Anderson JL (1990) Rod visual fields in cone-rod degeneration. Comparisons to retinitis pigmentosa. Invest Ophthalmol Vis Sci 31:2288–2299
Breton ME, Montzka DP, Brucker AJ, Quinn GE (1991) Electroretinogram interpretation in central retinal vein occlusion. Ophthalmology 98:1837–1844
Hood DC, Shady S, Birch DG (1994) Understanding changes in the b-wave of the ERG caused by heterogeneous receptor damage. Invest Ophthalmol Vis Sci 35:2477–2488
Velten IM, Horn FK, Korth M, Velten K (2001) The b-wave of the dark adapted flash electroretinogram in patients with advanced asymmetrical glaucoma and normal subjects. Br J Ophthalmol 85:403–409
Gabrieli CB, Regine F, Vingolo EM, Rispoli E, Fabbri A, Isidori A (2001) Subjective visual halos after sildenafil (Viagra) administration: electroretinographic evaluation. Ophthalmology 108:877–881
Gabrieli CB, Regine F, Vingolo EM, Rispoli E, Isidori A (2003) Acute electroretinographic changes during sildenafil (viagra) treatment for erectile dysfunction. Doc Ophthalmol 107:111–114
Ziemssen F, Lüke M, Messias A, Beutel J, Tatar O, Zrenner E, Bartz-Schmidt KU, Tuebingen Bevacizumab Study Group (2008) Safety monitoring in bevacizumab (Avastin) treatment: retinal function assessed by psychophysical (visual fields, colour vision) and electrophysiological (ERG/EOG) tests in two subgroups of patients. Int Ophthalmol 28:101–109
Stahl A, Feltgen N, Fuchs A, Bach M (2009) Electrophysiological evaluation of retinal photoreceptor function after repeated bevacizumab injections. Doc Ophthalmol 118:81–88
Varghese SB, Reid JC, Hartmann EE, Keyser KT (2011) The effects of nicotine on the human electroretinogram. Invest Ophthalmol Vis Sci 52:9445–9951
Constable PA, Gaigg SB, Bowler DM, Jägle H, Thompson DA (2016) Full-field electroretinogram in autism spectrum disorder. Doc Ophthalmol 132:83–99
Naka KI, Rushton WA (1966) S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555
Dawson WW, Trick GL, Litzkow CA (1979) Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 18:988–991
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Wali N, Leguire LE (1992) The photopic hill: a new phenomenon of the light adapted electroretinogram. Doc Ophthalmol 80:335–345
Rufiange M, Dassa J, Dembinska O, Koenekoop RK, Little JM, Polomeno RC, Dumont M, Chemtob S, Lachapelle P (2003) The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Res 43:1405–1412
Ueno S, Kondo M, Niwa Y, Terasaki H, Miyake Y (2004) Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Birch DG, Anderson JL (1992) Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol 110:1571–1576
Tang J, Edwards T, Crowston JG, Sarossy M (2014) The test–retest reliability of the photopic negative response (PhNR). Transl Vis Sci Technol 3:1
Weleber RG (1981) The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci 20:392–399
Mortlock KE, Binns AM, Aldebasi YH, North RV (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121:123–134
Kundra H, Park JC, McAnany JJ (2016) Comparison of photopic negative response measurements in the time and time-frequency domains. Doc Ophthalmol 133:91–98
Wu Z, Hadoux X, Fan Gaskin JC, Sarossy MG, Crowston JG (2016) Measuring the photopic negative response: viability of skin electrodes and variability across disease severities in glaucoma. Transl Vis Sci Technol 5:13
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Usha Govindarajulu for assistance with some of the statistical analysis included in this study.
Funding
National Institutes of Health provided financial support in the form of an institutional training grant (National Eye Institute grant T35 EY020481) to State University of New York College of Optometry. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
No animals used in this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Joshi, N.R., Ly, E. & Viswanathan, S. Intensity response function of the photopic negative response (PhNR): effect of age and test–retest reliability. Doc Ophthalmol 135, 1–16 (2017). https://doi.org/10.1007/s10633-017-9591-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9591-0