Abstract
Purpose
Previous work has suggested that the retinal degeneration mutant rd8 mouse lacks an electroretinographic (ERG) phenotype until about 9 months of age. We evaluated the ERG phenotype of these mice by measuring both conventional ERG responses and scotopic threshold responses.
Methods
Groups of 4-month-old wild-type (WT) and mutant (rd8) mice were anesthetized and tested for mass retinal responses (ERGs) to several types of visual stimuli. Scotopic threshold responses were accumulated with brief scotopic flashes at a series of very dim intensities. Dark-adapted (scotopic) and light-adapted (photopic) responses to brief flashes at a series of higher intensities were recorded, along with long flashes and random modulations of light levels under photopic conditions.
Results
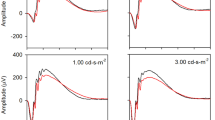
Negative scotopic threshold responses (nSTRs) had lower amplitudes in rd8 mice compared to WTs. Positive scotopic threshold responses were similar in the two groups. With the more intense stimuli, a- and c-wave amplitudes were smaller in rd8 mice. Both scotopic and photopic b-wave amplitudes tended to be larger in rd8 mice, though generally not significantly.
Conclusions
The striking decrease in nSTR amplitudes was surprising, given that the main retinal effects of the rd8 mutation occur in the outer retina, at the external limiting membrane. The primary source of nSTRs in mice is thought to be at the amacrine cell level in the inner retina. Investigation of how this mutation leads to inner retinal dysfunction might reveal unexpected aspects of retinal cell biology and circuitry.




Similar content being viewed by others
References
Alves CH, Pellissier LP, Wijnholds J (2014) The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog Retin Eye Res 40:35–52. doi:10.1016/j.preteyeres.2014.01.001
Paniagua AE, Herranz-Martin S, Jimeno D, Jimeno AM, Lopez-Benito S, Arevalo JC, Velasco A, Aijon J, Lillo C (2015) CRB2 completes a fully expressed Crumbs complex in the retinal pigment epithelium. Sci Rep 5:14504. doi:10.1038/srep14504
Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, Bechtold L, Haider NB, Tepass U, Heckenlively JR, Chang B, Naggert JK, Nishina PM (2003) CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet 12:2179–2189. doi:10.1093/hmg/ddg232
Zhao M, Andrieu-Soler C, Kowalczuk L, Paz Cortés M, Berdugo M, Dernigoghossian M, Halili F, Jeanny J-C, Goldenberg B, Savoldelli M, El Sanharawi M, Naud M-C, van Ijcken W, Pescini-Gobert R, Martinet D, Maass A, Wignholds J, Crisanti P, Rivolta C, Behar-Cohen F (2015) A new CRB1 rat mutation links Muller glial cells to retinal telangiectasia. J Neurosci 35:6093–6106. doi:10.1523/JNEUROSCI.3412-14.2015
Jacobson SG, Cideciyan AV, Aleman TS, Pianta MJ, Sumaroka A, Schwartz SB, Smilko EE, Milam AH, Sheffield VC, Stone EM (2003) Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet 12:1073–1078
Mattapallil MJ, Wawrousek EF, Chan C-C, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6 N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci 53:2921–2927. doi:10.1167/iovs.12-9662
Lawrance AK, Racine J, Deng L, Wang X, Lachapelle P, Rozen R (2011) Complete deficiency of methylenetetrahydrofolate reductase in mice is associated with impaired retinal function and variable mortality, hematological profiles, and reproductive outcomes. J Inherit Metab Dis 34:147–157. doi:10.1007/s10545-010-9127-1
Markand S, Saul A, Roon P, Prasad P, Martin P, Rozen R, Ganapaty V, Smith SB (2015) Retinal ganglion cell loss and mild vasculopathy in methylene tetrahydrofolate reductase (Mthfr) deficient mice: a model of mild hyperhomocysteinemia. Invest Ophthalmol Vis Sci 56:2684–2695. doi:10.1167/iovs.14-16190
Markand S, Saul A, Tawfik A, Cui X, Rozen R, Smith SB (2016) Mthfr as a modifier of the retinal phenotype of Crb1rd8/rd8 mice. Exp Eye Res 145:164–172. doi:10.1016/j.exer.2015.11.013
Aleman TS, Cideciyan AV, Aguirre GK, Huang WC, Mullins CL, Roman AJ, Sumaroka A, Olivares MB, Tsai FF, Schwartz SB, Vandenberghe LH, Limberis MP, Stone EM, Bell P, Wilson JM, Jacobson SG (2011) Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model. Invest Ophthalmol Vis Sci 52:6898–6910. doi:10.1167/iovs.11-7701
Sieving PA, Frishman LJ, Steinberg RH (1986) Scotopic threshold response of proximal retina in cat. J Neurophysiol 56:1049–1061
Saszik SM, Robson JG, Frishman LJ (2002) The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol 543:899–916
Frishman LJ (2006) Origins of the electroretinogram. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision, 2nd edn. MIT Press, Cambridge, pp 139–183
Oakley B, Green DG (1976) Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol 39:1117–1133
Yanase J, Ogawa H (1997) Effects of halothane and sevoflurane on the electroretinogram of dogs. Am J Veterinary Res 58:904–909
Saul AB, Still AE. (2016) Multifocal electroretinography in the presence of temporal and spatial correlations and eye movements. Vision 1:3 (http://www.mdpi.com/2411-5150/1/1/3). doi:10.3390/vision1010003
Luhmann UF, Carvalho LS, Kleine Holthaus SM, Cowing JA, Greenaway S, Chu CJ, Ali RR (2015) The severity of retinal pathology in homozygous CRB1rd8/rd8 mice is dependent on additional genetic factors. Hum Mol Genet 24:128–141. doi:10.1093/hmg/ddu424
Moshiri A, Gonzalez E, Tagawa K, Maeda H, Wang M, Frishman LJ, Wang SW (2008) Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Developmental Biology. 316:214–227. doi:10.1016/j.ydbio.2008.01.015
Abd-El-Barr MM, Pennesi ME, Saszik SM, Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM (2009) Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol 102:1945–1955. doi:10.1152/jn.00142.2009
Frishman LJ, Steinberg RH (1989) Intraretinal analysis of the threshold dark-adapted ERG of cat retina. J Neurophysiol 61:1221–1232
Funding
National Institutes of Health/National Eye Institute provided financial support in the form of grants 2R01EY012830, R01EY014560. The James and Jean Culver Vision Discovery Institute also provided financial support. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Statement of human rights
No human subjects were tested in the present study.
Statement on the welfare of animals
All procedures were approved by the Institutional Animal Care and Use Committee of Augusta University, and followed the ARVO guidelines for the use of animals in ophthalmic and visual research.
Informed consent
No humans were tested in this study.
Rights and permissions
About this article
Cite this article
Saul, A.B., Cui, X., Markand, S. et al. Detailed electroretinographic findings in rd8 mice. Doc Ophthalmol 134, 195–203 (2017). https://doi.org/10.1007/s10633-017-9585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9585-y