Abstract
Purpose
To determine whether significant correlations existed between the morphological and functional parameters of the macular region of eyes with open-angle glaucoma (OAG).
Methods
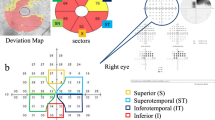
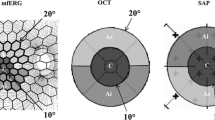
Forty eyes of 40 OAG patients were studied. The morphological parameters were obtained by optical coherence tomography (OCT), and the functional parameters were acquired by automated Humphrey Field Analyzer (HFA) and multifocal electroretinograms (mfERGs). All of the tests were performed within 6 months of each other. The retinal thickness was determined by OCT in the nine Early Treatment of Diabetic Retinopathy Study (ETDRS) sectors of the macula, the fovea, and the four quadrants of the inner and an outer ring. The amplitudes of the second-order kernel responses of the mfERGs in the central 5° including the amplitude ratio of the nasal to temporal hemispheres (N/T amplitude ratio) were analyzed. The total mean deviation of the HFA corresponding to each OCT region was measured. The correlation between the different parameters was determined by coefficients of correlation and linear regression analyses.
Results
The N/T amplitude ratio of the second-order kernel responses of the mfERGs was significantly correlated with the retinal thickness in the inferior quadrant (r = −0.44; P = 0.004). There was a significant correlation between the N/T amplitude ratio and the threshold in the superior quadrant measured by the HFA Central 10-2 program (r = −0.40; P = 0.011) and also between the N/T amplitude ratio and the total deviation in the superior quadrant (r = −0.40; P = 0.010). There were significant correlations between the inferior retinal thickness and the average threshold and the TD in superior (r = 0.70, P < 0.001; r = 0.692, P < 0.001, respectively), nasal (r = 0.53, P < 0.001; r = 0.53, P < 0.001, respectively), and temporal (r = 0.46, P = 0.003; r = 0.44, P = 0.004, respectively) quadrants.
Conclusions
Functional glaucomatous changes determined by mfERGs and perimetry are significantly correlated with the morphological changes determined by OCT.



Similar content being viewed by others
References
Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, Witt KA (1991) Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 109:77–83
Ly T, Gupta N, Weinreb RN, Kaufman PL, Yücel YH (2011) Dendrite plasticity in the lateral geniculate nucleus in primate glaucoma. Vision Res 51:243–250
Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A (1992) An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology 99:19–28
Zeyen TG, Caprioli J (1993) Progression of disc and field damage in early glaucoma. Arch Ophthalmol 111:62–65
Park SB, Sung KR, Kang SY, Kim KR, Kook MS (2009) Comparison of glaucoma diagnostic capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol 127:1603–1609
Zeimer R, Asrani S, Zou S, Quigley H, Jampel H (1998) Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology 105:224–231
Lederer DE, Schuman JS, Hertzmark E, Heltzer J, Velazques LJ, Fujimoto JG, Mattox C (2003) Analysis of macular volume in normal and glaucomatous eyes using optical coherence tomography. Am J Ophthalmol 135:838–843
Guedes V, Schuman JS, Hertzmark E, Wollstein G, Correnti A, Mancini R, Lederer D, Voskanian S, Velazquez L, Pakter HM, Pedut-Kloizman T, Fujimoto JG, Mattox C (2003) Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 110:177–189
Greenfield DS, Bagga H, Knighton RW (2003) Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol 121:41–46
Parikh RS, Parikh SR, Thomas R (2010) Diagnostic capability of macular parameters of Stratus OCT 3 in detection of early glaucoma. Br J Ophthalmol 94:197–201
Velten IM, Korth M, Horn FK (2001) The a-wave of the dark adapted electroretinogram in glaucomas: are photoreceptors affected? Br J Ophthalmol 85:397–402
Sehi M, Pinzon-Plazas M, Feuer WJ, Greenfield DS (2009) Relationship between pattern electroretinogram, standard automated perimetry, and optic nerve structural assessments. J Glaucoma 18:608–617
Machida S, Tamada K, Oikawa T, Yokoyama D, Kaneko M, Kurosaka D (2010) Sensitivity and specificity of photopic negative response of focal electoretinogram to detect glaucomatous eyes. Br J Ophthalmol 94:202–208
Vincent A, Shetty R, Devi SA, Kurian MK, Balu R, Shetty B (2010) Functional involvement of cone photoreceptors in advanced glaucoma: a multifocal electroretinogram study. Doc Ophthalmol 121:21–27
Hood DC, Greenstein VC, Holopigian K, Bauer R, Firoz B, Liebmann JM, Odel JG, Ritch R (2000) An attempt to detect glaucomatous damage to the inner retina with the multifocal ERG. Invest Ophthalmol Vis Sci 41:1570–1579
Asano E, Mochizuki K, Sawada A, Nagasaka E, Kondo Y, Yamamoto T (2007) Decreased nasal-temporal asymmetry of the second-order kernel response of multifocal electroretinograms in eyes with normal-tension glaucoma. Jpn J Ophthalmol 51:379–389
Nakamura H, Hangai M, Mori S, Hirose F, Yoshimura N (2011) Hemispherical focal macular photopic negative response and macular inner retinal thickness in open-angle glaucoma. Am J Ophthalmol 151:494–506
Falsini B, Marangoni D, Salgarello T, Stifano G, Montrone L, Campagna F, Aliberti S, Balestrazzi E, Colotto A (2008) Structure–function relationship in ocular hypertension and glaucoma: interindividual and interocular analysis by OCT and pattern ERG. Graefes Arch Clin Exp Ophthalmol 246:1153–1162
Vaegan, Graham SL, Goldberg I, Buckland L, Hollows FC (1995) Flash and pattern electroretinogram changes with optic atrophy and glaucoma. Exp Eye Res 60:697–706
Weiner A, Ripkin DJ, Patel S, Kaufman SR, Kohn HD, Weidenthal DT (1998) Foveal dysfunction and central visual field loss in glaucoma. Arch Ophthalmol 116:1169–1174
Bearse MA Jr, Sim D, Sutter EE, Stamper R, Leiberman M (1996) Application of the multi-focal ERG to glaucoma. Invest Ophthalmol Vis Sci 37:S511
Hasegawa S, Takagi M, Usui T, Takada R, Abe H (2000) Waveform changes of the first-order multifocal electroretinogram in patients with glaucoma. Invest Ophthalmol Vis Sci 41:1597–1603
Fortune B, Johnson CA, Cioffi GA (2001) The topographic relationship between multifocal electroretinographic and behavioral perimetric measures of function in glaucoma. Optom Vis Sci 78:206–214
Jonas JB, Schneider U, Naumann GO (1992) Count and density of human retinal photoreceptors. Graefes Arch Clin Exp Ophthalmol 230:505–510
Curcio CA, Sloan KR, Kalina RE, Hendrickson AE (1990) Human photoreceptor topography. J Comp Neurol 292:497–523
Chan HH, Brown B (2000) Pilot study of the multifocal electroretinogram in ocular hypertension. Br J Ophthalmol 84:1147–1153
Chan HL, Brown B (1999) Multifocal ERG changes in glaucoma. Ophthalmic Physiol 19:306–316
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM, International Society For Clinical Electrophysiology of Vision (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124:1–13
Sutter EE, Bearse MA (1999) The optic nerve head component of the human ERG. Vision Res 39:419–436
Hood DC, Raza AS (2011) Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed Opt Express 2:1097–1105
Luo X, Patel NB, Harwerth RS, Frishman LJ (2011) Loss of the low-frequency component of the global-flash multifocal electroretinogram in primate eyes with experimental glaucoma. Invest Ophthalmol Vis Sci 52:3792–3804
Fortune B, Bearse MA Jr, Cioffi GA, Johnson CA (2002) Selective loss of an oscillatory component from temporal retinal multifocal ERG responses in glaucoma. Invest Ophthalmol Vis Sci 43:2638–2647
Hood DC, Zhang X (2000) Multifocal ERG and VEP responses and visual fields: comparing disease-related changes. Doc Ophthalmol 100:115–137
Palmowski-Wolfe AM, Allgayer RJ, Vernaleken B, Schötzau A, Ruprecht KW (2006) Slow-stimulated multifocal ERG in high- and normal-tension glaucoma. Doc Ophthalmol 112:157–168
Chu PH, Chan HH, Brown B (2006) Glaucoma detection is facilitated by luminance modulation of the global flash multifocal electroretinogram. Invest Ophthalmol Vis Sci 47:929–937
Chu PH, Ng YF, Ting PW, Lung JC, Ho WC, So KF, To CH, Chan HH (2011) Effect of inner retinal dysfunction on slow double-stimulation multifocal electroretinogram. Br J Ophthalmol 95:1597–1602
Hood DC (2000) Assessing retinal function with the multifocal technique. Prog Retin Eye Res 19:607–646
Kawabata H, Adachi-Usami E (1997) Multifocal electroretinogram in myopia. Invest Ophthalmol Vis Sci 38:2844–2851
Chan HL, Mohidin N (2003) Variation of multifocal electroretinogram with axial length. Ophthalmic Physiol Opt 23:133–140
Chen JC, Brown B, Schmid KL (2006) Delayed mfERG responses in myopia. Vision Res 46:1221–1229
Luu CD, Lau AM, Lee SY (2006) Multifocal electroretinogram in adults and children with myopia. Arch Ophthalmol 124:328–334
Suzuki Y, Iwase A, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, Inoue Y, Kitazawa Y, Tajimi Study Group (2006) Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology 113:1613–1617
Glovinsky Y, Quigley HA, Pease ME (1993) Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 34:395–400
Wollstein G, Schuman JS, Price LL, Aydin A, Beaton SA, Stark PC, Fujimoto JG, Ishikawa H (2004) Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol 138:218–225
Legarreta JE, Gregori G, Punjabi OS, Knighton RW, Lalwani GA, Puliafito CA (2008) Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging 39(Suppl):S43–S49
Sull AC, Vuong LN, Price LL, Srinivasan VJ, Gorczynska I, Fujimoto JG, Schuman JS, Duker JS (2010) Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina 30:235–245
Chu PH, Ng YF, To CH, So KF, Brown B, Chan HH (2012) Luminance-modulated adaptation in the global flash mfERG: a preliminary study of early retinal functional changes in high-risk glaucoma patients. Graefes Arch Clin Exp Ophthalmol 250:261–270
Kotera Y, Hangai M, Hirose F, Mori S, Yoshimura N (2011) Three-dimensional imaging of macular inner structures in glaucoma by using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 52:1412–1421
Sato A, Fukui E, Ohta K (2010) Retinal thickness of myopic eyes determined by spectralis optical coherence tomography. Br J Ophthalmol 94:1624–1628
Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, Varma R, Huang D (2009) Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 116:2305–2314
Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, Liebmann JM, Ritch R, Hood DC (2011) Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol 129:1529–1536
Bowd C, Tafreshi A, Zangwill LM, Medeiros FA, Sample PA, Weinreb RN (2011) Pattern electroretinogram association with spectral domain-OCT structural measurements in glaucoma. Eye 25:224–232
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hori, N., Komori, S., Yamada, H. et al. Assessment of macular function of glaucomatous eyes by multifocal electroretinograms. Doc Ophthalmol 125, 235–247 (2012). https://doi.org/10.1007/s10633-012-9351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-012-9351-0