Abstract
Background
Acute pancreatitis (AP) is a common disease of the digestive system. The mechanism of hyperbaric oxygen (HBO) therapy for AP is not completely clear.
Aims
This study investigated the effects of HBO in AP and whether it acts through the mitochondria-mediated apoptosis pathway.
Methods
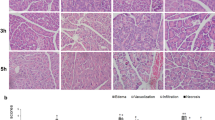
Eighty male Sprague–Dawley rats were randomly assigned to four groups: control (8 rats), sham (24 rats), AP (24 rats), or AP + HBO (24 rats). AP was induced by ligating the pancreatic duct. The AP + HBO group was given HBO therapy starting at 6 h postinduction. Eight rats in each group were killed on days 1, 2, and 3 postinduction to assess pancreatic injury, mitochondrial membrane potential, ATP level, and expression levels of BAX, Bcl-2, caspase-3, caspase-9, and PARP in pancreatic tissue and blood levels of amylase, lipase, and pro-inflammatory cytokines.
Results
HBO therapy alleviated the severity of AP and decreased histopathological scores and levels of serum amylase, lipase, and pro-inflammatory cytokines. Compared to AP induction alone, HBO therapy increased expression of the apoptotic protein BAX, caspase-3, caspase-9, and PARP and ATP levels in tissues and decreased antiapoptotic protein Bcl-2 expression levels and the mitochondrial membrane potential on the first day; the results on the second day were partly consistent with those on the first day, while there was no obvious difference on the third day.
Conclusions
HBO therapy could induce caspase-dependent apoptosis in AP rats to alleviate pancreatitis, which was possibly triggered by mitochondrial apoptosis pathway regulation of Bcl-2 family members.







Similar content being viewed by others
Change history
09 May 2020
The original version of the article unfortunately contained an error in the legend of Figure��2b and 2c.
References
Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55.
Lankisch PG, Apte M, Banks PA. Acute Pancreatitis. Lancet. 2015;386:85–96.
Tenner S, Baillie J, DeWitt J, Vege SS. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415. https://doi.org/10.1038/ajg.2013.218.
Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–G196.
Leindler L, Morschl É, László F, Mándi Y, Takács T, et al. Importance of cytokines, nitric oxide, and apoptosis in the pathological process of necrotizing pancreatitis in rats. Pancreas. 2004;29:157–161.
Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med. 2004;8:402–409.
Mareninova OA, Sung KF, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381.
Frossard JL, Rubbia-Brandt L, Wallig MA, et al. Severe acute pancreatitis and reduced acinar cell apoptosis in the exocrine pancreas of mice deficient for the Cx32 gene. Gastroenterology. 2003;124:481–493.
Tejada S, Batle JM, Ferrer MD, et al. Therapeutic effects of hyperbaric oxygen in the process of wound healing. Curr Pharm Des. 2019;25:1682–1693. https://doi.org/10.2174/1381612825666190703162648.
Halbach JL, Prieto JM, Wang AW, et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis. Am J Physiol Regul Integr Comp Physiol. 2019;317:R160–R168.
Bian H, Huang L, Li B, et al. The arousal effect of hyperbaric oxygen through orexin/hypocretin an upregulation on ketamine/ethanol-induced unconsciousness in male rats. J Neurosci Res. 2020;98:201–211. https://doi.org/10.1002/jnr.24414.
Inal V, Mas MR, Isik AT, et al. A new combination therapy in severe acute pancreatitis–hyperbaric oxygen plus 3-aminobenzamide: an experimental study. Pancreas. 2015;44:326–330.
Bai X, Sun B, Pan S, et al. Down-regulation of hypoxia-inducible factor-1alpha by hyperbaric oxygen attenuates the severity of acute pancreatitis in rats. Pancreas. 2009;38:515–522.
Yu QH, Zhang PX, Liu Y, Liu W, Yin N. Hyperbaric oxygen preconditioning protects the lung against acute pancreatitis induced injury via attenuating inflammation and oxidative stress in a nitric oxide dependent manner. Biochem Biophys Res Commun. 2016;478:93–100.
Bai X, Song Z, Zhou Y, et al. The apoptosis of peripheral blood lymphocytes promoted by hyperbaric oxygen treatment contributes to attenuate the severity of early stage acute pancreatitis in rats. Apoptosis Int J Prog Cell Death. 2014;19:58–75. https://doi.org/10.1007/s10495-013-0911-x.
Meyerholz DK, Samuel I. Morphologic characterization of early ligation-induced acute pancreatitis in rats. Am J Surg. 2007;194:652–658. https://doi.org/10.1016/j.amjsurg.2007.07.014.
Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. https://doi.org/10.1097/00000658-199201000-00007.
Richter C, Schweizer M, Cossarizza A, Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378:107–110. https://doi.org/10.1016/0014-5793(95)01431-4.
Cavalcante GC, Schaan AP, Cabral GF, et al. A cell’s fate: an overview of the molecular biology and genetics of apoptosis. Int J Mol Sci. 2019;. https://doi.org/10.3390/ijms20174133.
Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517–1525.
Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8:10–25. https://doi.org/10.4292/wjgpt.v8.i1.10.
Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. TNF-alpha as a therapeutic target in acute pancreatitis–lessons from experimental models. Sci World J. 2007;7:431–448. https://doi.org/10.1100/tsw.2007.98.
Zhang FH, Sun YH, Fan KL, et al. Protective effects of heme oxygenase-1 against severe acute pancreatitis via inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. BMC Gastroenterol. 2017;17:100. https://doi.org/10.1186/s12876-017-0651-4.
Zhang H, Yang W, Li Y, et al. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. Int Immunopharmacol. 2018;56:18–28. https://doi.org/10.1016/j.intimp.2018.01.011.
Christophi C, Millar I, Nikfarjam M, Muralidharan V, Malcontenti-Wilson C. Hyperbaric oxygen therapy for severe acute pancreatitis. J Gastroenterol Hepatol. 2007;22:2042–2046.
Gw B, Cs P. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12:7087–7096.
Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. https://doi.org/10.1159/000085265.
Koh SL, Tan JW, Muralidharan V, Christophi C. The effect of hyperbaric oxygen on apoptosis and proliferation in severe acute pancreatitis. HPB Off J Int Hepato Pancreato Biliary Assoc. 2009;11:629–637.
Gukovskaya AS, Perkins PE, Zaninovic VJ, et al. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996;110:875–884.
Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–2125.
Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429.
Kim JS, Qian T. Lemasters JJ Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503.
Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163.
Galluzzi L, Maiuri MC, Vitale I, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. https://doi.org/10.1038/sj.cdd.4402148.
Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299:214–215. https://doi.org/10.1126/science.1081274.
Zhou Z, Daugherty WP, Sun D, et al. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J Neurosurg. 2007;106(4):687–694.
Mei LH, Yang G, Fang F. Hyperbaric oxygen combined with 5-aminolevulinic acid photodynamic therapy inhibited human squamous cell proliferation. Biol Pharm Bull. 2019;42:394–400.
Harada H, Grant S. Apoptosis regulators. Rev Clin Exp Hematol. 2003;7:117–138.
Lebedeva IV, Sarkar D, Su ZZ, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. https://doi.org/10.1038/sj.onc.1206891.
Zn O, Cl M, Sj K. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619.
Zhang XP, Tian H, Lu B, et al. Tissue microarrays in pathological examination of apoptotic acinar cells induced by dexamethasone in the pancreas of rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:527–536.
Inuzuka K, Unno N, Yamamoto N, et al. Effect of hyperbarically oxygenated-perfluorochemical with University of Wisconsin solution on preservation of rat small intestine using an original pressure-resistant portable apparatus. Surgery. 2007;142:57–66. https://doi.org/10.1016/j.surg.2007.03.002.
Acknowledgments
We thank Mrs. Mingming Xiao for expert technical assistance on histopathological score about pancreas. We are also grateful to the Pathology Laboratory of China Medical University and Key Laboratory of Immunodermatology, Ministry of Health, Ministry of Education, No. 1 Hospital of China Medical University, for its technical assistance.
Funding
This study was funded by the Natural Science Foundation of Liaoning Province (Grant Number 20170540526).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, H., Ge, B., Yuan, Y. et al. Hyperbaric Oxygen Ameliorated Acute Pancreatitis in Rats via the Mitochondrial Pathway. Dig Dis Sci 65, 3558–3569 (2020). https://doi.org/10.1007/s10620-020-06070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06070-3