Abstract
Background
Inflammatory bowel disease (IBD) is a common disorder of chronic intestinal inflammation that can be caused by the disruption of intestinal immune homeostasis.
Aim
We aimed to evaluate the role of enhancer of zeste homolog 2 (EZH2) in the inflammatory response and explore the association between EZH2 and necroptosis in human epithelial colorectal adenocarcinoma cell lines.
Methods
In both in vitro and in vivo models, expression of EZH2 in intestinal tissues was verified by histology. The expression of inflammatory cytokines in cell lines treated with EZH2 siRNA with or without stimulus was analyzed by quantitative real-time polymerase chain reaction. An intestinal necroptosis cell model was established to elucidate whether EZH2 is involved in necroptosis.
Results
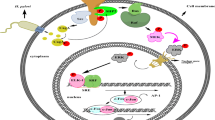
Our present data indicated that EZH2 expression was decreased in in vitro and in vivo models and in patients with inflammatory bowel disease. EZH2 downregulation increased the expression of inflammatory factors, including TNF-α, IL-8, IL-17, CCL5, and CCL20 in a Caco-2 cell model. The JNK pathway was activated with the reduction of EZH2. In the necroptosis model, downregulation of EZH2 was detected with the upregulation of necroptotic markers RIP1 and RIP3. In addition, EZH2 knockdown with siRNA increased p-JNK and p-c-Jun.
Conclusion
Our data suggest that EZH2 plays an important role in the development of intestinal inflammation and necroptosis. Hence, EZH2 could be a potential therapeutic target for IBD.




Similar content being viewed by others
References
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153.
Scarpa M, Kessler S, Sadler T, et al. The epithelial danger Signal IL-1 alpha is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol. 2015;185:1624–1637.
Kim KH, Roberts CWM. Targeting EZH2 in cancer. Nat Med. 2016;22:128–134.
Fillmore CM, Xu CX, Desai PT, et al. EZH2 inhibition sensitizes BRG1 and EGFR mutant lung tumours to TopoII inhibitors. Nature. 2015;520:239–242.
Sarmento OF, Svingen PA, Xiong YN, et al. The role of the histone methyltransferase enhancer of zeste homolog 2 (EZH2) in the pathobiological mechanisms underlying inflammatory bowel disease (IBD). J Biol Chem. 2017;292:706–722.
Tsou PS, Coit P, Kilian NC, Sawalha AH. EZH2 modulates the DNA methylome and controls T cell adhesion through junctional adhesion molecule a in lupus patients. Arthritis Rheumatol. 2018;70:98–108.
Seo J, Lee EW, Sung H, et al. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol. 2016;18:291–302.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320.
Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071.
Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339.
Wu XN, Yang ZH, Wang XK, et al. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–1720.
Humphries F, Yang S, Wang B, Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2015;22:225–236.
Zhe-Wei S, Li-Sha G, Yue-Chun L. The role of necroptosis in cardiovascular disease. Front Pharmacol. 2018;9:721.
Han CH, Guan ZB, Zhang PX, et al. Oxidative stress induced necroptosis activation is involved in the pathogenesis of hyperoxic acute lung injury. Biochem Biophys Res Commun. 2018;495:2178–2183.
Guo YL, Wu XX, Wu Q, Lu YF, Shi JS, Chen XP. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicol Appl Pharmacol. 2018;344:35–45.
Liu YF, Peng JJ, Sun TY, et al. Epithelial EZH2 serves as an epigenetic determinant in experimental colitis by inhibiting TNF alpha-mediated inflammation and apoptosis. Proc Natl Acad Sci U S A. 2017;114:E3796–E3805.
Zhu H, Wan X, Li J, et al. Computational prediction and validation of BAHD1 as a novel molecule for ulcerative colitis. Sci Rep. 2015;5:12227.
Van De Walle J, Hendrickx A, Romier B, Larondelle Y, Schneider YJ. Inflammatory parameters in Caco-2 cells: effect of stimuli nature, concentration, combination and cell differentiation. Toxicol Vitro. 2010;24:1441–1449.
Matsuzawa-Ishimoto Y, Shono Y, Gomez LE, et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J Exp Med. 2017;214:3687–3705.
Negroni A, Colantoni E, Pierdomenico M, et al. RIP3 AND pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells. Dig Liver Dis. 2017;49:1201–1210.
Sergent T, Parys M, Garsou S, Pussemier L, Schneider YJ, Larondelle Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol Lett. 2006;164:167–176.
Assi K, Pillai R, Gomez-Munoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121.
Kobayashi K, Arimura Y, Goto A, et al. Therapeutic implications of the specific inhibition of causative matrix metalloproteinases in experimental colitis induced by dextran sulphate sodium. J Pathol. 2006;209:376–383.
Gautheron J, Vucur M, Reisinger F, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. Embo Mol Med. 2014;6:1062–1074.
Sun W, Wu XX, Gao HW, et al. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free Radic Biol Med. 2017;108:433–444.
Liu ZY, Wu B, Guo YS, et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5:3174–3185.
Maleszewska M, Gjaltema RA, Krenning G, Harmsen MC. Enhancer of zeste homolog-2 (EZH2) methyltransferase regulates transgelin/smooth muscle-22alpha expression in endothelial cells in response to interleukin-1beta and transforming growth factor-beta2. Cell Signal. 2015;27:1589–1596.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185.
Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342.
Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, Suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139.
Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol. 2014;29:502–510.
Nishida A, Hidaka K, Kanda T, et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflammy Bowel Dis. 2016;22:303–314.
Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49.
Lee J, Mo J-H, Shen C, Rucker AN, Raz E. Toll-like receptor signaling in intestinal epithelial cells contributes to colonic homoeostasis. Curr Opin Gastroenterol. 2007;23:27–31.
Arifuzzaman S, Das A, Kim SH, et al. Selective inhibition of EZH2 by a small molecule inhibitor regulates microglial gene expression essential for inflammation. Biochem Pharmacol. 2017;137:61–80.
Rosillo MA, Sanchez-Hidalgo M, Cardeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol. 2011;82:737–745.
Wang Y, Li Z, Zhang H, et al. HIF-1 alpha and HIF-2 alpha correlate with migration and invasion in gastric cancer. Cancer Biol Ther. 2010;10:376–382.
Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–882.
Liu J, Chang F, Li F, et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun. 2015;463:262–267.
Santabarbara-Ruiz P, Lopez-Santillan M, Martinez-Rodriguez I, et al. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during drosophila regeneration. PLoS Genet. 2015;11:e1005595.
Palit S, Kar S, Sharma G, Das PK. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J Cell Physiol. 2015;230:1729–1739.
Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao RK. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J. 2015;465:503–515.
Pierdomenico M, Negroni A, Stronati L, et al. necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol. 2014;109:279–287.
Berger SB, Kasparcova V, Hoffman S, et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key Regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480.
Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNF alpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37.
Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–136.
Arosh JA, Lee J, Starzinski-Powitz A, Banu SK. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 modulates DNA methylation and histone modification machinery proteins in human endometriotic cells. Mol Cell Endocrinol. 2015;409:51–58.
Gong Y, Huo L, Liu P, et al. Polycomb group protein EZH2 is frequently expressed in inflammatory breast cancer and is predictive of worse clinical outcome. Cancer. 2011;117:5476–5484.
Webb LM, Guerau-de-Arellano M. Emerging role for methylation in multiple sclerosis: beyond DNA. Trends Mol Med. 2017;23:546–562.
Acknowledgements
The authors would like to thank Jie Zhang, Yuwei Zhang and the teachers as well as students of the Laboratory of gastroenterology, The First Affiliated Hospital, College of Medicine, Zhejiang University, for helpful technical assistance.
Funding
Supported by the National Natural Science Foundation of China (81600413), co-sponsored project of province and ministry of Zhejiang Province of China under Grant (WKJ-ZJ-1516), and Natural Science Foundation of Zhejiang Province (LQ16H030001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lou, X., Zhu, H., Ning, L. et al. EZH2 Regulates Intestinal Inflammation and Necroptosis Through the JNK Signaling Pathway in Intestinal Epithelial Cells. Dig Dis Sci 64, 3518–3527 (2019). https://doi.org/10.1007/s10620-019-05705-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05705-4