Abstract
Background
Normal hepatocytes exhibit low-affinity hexokinase (glucokinase [HKIV]), but during oncogenesis, there is a switch from HKIV to HKII expression. The aims of this study were to compare the immunoexpression of HKII in non-dysplastic cirrhosis (NDC), liver cell change/dysplasia in cirrhosis (LCD), HCC, and normal liver control tissues, and to correlate HKII expression with clinical and histopathological parameters.
Design
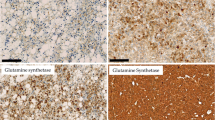
Immunohistochemistry was performed on a liver cancer progression tissue array consisting of specimens from explants with cirrhosis, including 45 tissue samples with HCC, 108 without HCC, 143 with LCD, and 8 normal liver control tissues. HKII expression was quantified as positive pixel counts/square millimeter (ppc/mm2) by image analysis.
Results
There was a stepwise increase in HKII level from normal liver tissue to NDC, to LCD, and to HCC (p = 0.001). HKII levels were significantly higher in areas of LCD versus NDC (p ≤ 0.001), and in LCD and HCC versus NDC (p = 0.007). HKII levels were similar in LCD and HCC (p = 0.124). HKII levels were higher in grade 2–4 versus grade 1 HCCs (p = 0.044), and in pleomorphic versus non-pleomorphic HCC variants (p = 0.041). Higher levels of HKII expression in LCD and HCC versus NDC and in higher tumor grade remained significant in multivariate analysis.
Conclusions
Higher levels of HKII immunoexpression in LDC and HCC compared with NDC suggest that upregulation of HKII occurs during the process of hepatocarcinogenesis in humans. In HCC, higher levels of HKII are associated with more aggressive histological features.


Similar content being viewed by others
References
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491.
Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346.
El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 2002;36:S74–S83.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917.
Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832.
Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207, x–xi.
Takamatsu S, Noguchi N, Kudoh A, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:609–614.
Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766.
Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38.
Rosmorduc O, Fartoux L. HCC and NASH: how strong is the clinical demonstration? Clin Res Hepatol Gastroenterol. 2012;36:202–208.
Chu CA, Fujimoto Y, Igawa K, et al. Rapid translocation of hepatic glucokinase in response to intraduodenal glucose infusion and changes in plasma glucose and insulin in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G627–G634.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899.
Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci U S A. 1977;74:3735–3739.
Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696.
Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658.
Dombrowski F, Filsinger E, Bannasch P, Pfeifer U. Altered liver acini induced in diabetic rats by portal vein islet isografts resemble preneoplastic hepatic foci in their enzymic pattern. Am J Pathol. 1996;148:1249–1256.
Goel A, Mathupala SP, Pedersen PL. Glucose metabolism in cancer. Evidence that demethylation events play a role in activating type II hexokinase gene expression. J Biol Chem. 2003;278:15333–15340.
Mathupala SP, Rempel A, Pedersen PL. Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr. 1997;29:339–343.
Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol. 2002;29:443–453.
Engles JM, Quarless SA, Mambo E, Ishimori T, Cho SY, Wahl RL. Stunning and its effect on 3H-FDG uptake and key gene expression in breast cancer cells undergoing chemotherapy. J Nucl Med. 2006;47:603–608.
Kim JE, Ahn BC, Hwang MH, et al. Combined RNA interference of hexokinase II and (131)I-sodium iodide symporter gene therapy for anaplastic thyroid carcinoma. J Nucl Med. 2011;52:1756–1763.
Peng SY, Lai PL, Pan HW, Hsiao LP, Hsu HC. Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep. 2008;19:1045–1053.
Guzman G, Chan A, Chennuri R, Patel R, Hay N, Cotler S. High level of hexokinase 2 (HK2) is observed in diabetes (DM), biologically aggressive hepatocellular carcinomas (HCCs), and in the progression of HCCs. http://www.multiwebcast.com/easl/2011/nice/listing/by_poster#_t_189. e-poster European Association for the Study of the Liver, Nice, France, Jun 28–29, 2011.
Kwee SA, Hernandez B, Chan O, Wong L. Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: association with survival. PLoS ONE. 2012;7:e46591.
Gong L, Cui Z, Chen P, Han H, Peng J, Leng X. Reduced survival of patients with hepatocellular carcinoma expressing hexokinase II. Med Oncol. 2012;29:909–914.
Ruby SG. Protocol for the examination of specimens from patients with hepatocellular carcinoma and cholangiocarcinoma, including intrahepatic bile ducts. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 2000;124:41–45.
Park YN, Roncalli M. Large liver cell dysplasia: a controversial entity. J Hepatol. 2006;45:734–743.
Roncalli M, Borzio M, Tombesi MV, Ferrari A, Servida E. A morphometric study of liver cell dysplasia. Hum Pathol. 1988;19:471–474.
Tiniakos DG, Brunt EM. Proliferating cell nuclear antigen and Ki-67 labeling in hepatocellular nodules: a comparative study. Liver. 1999;19:58–68.
Gong L, Li YH, Su Q, Chu X, Zhang W. Clonality of nodular lesions in liver cirrhosis and chromosomal abnormalities in monoclonal nodules of altered hepatocytes. Histopathology. 2010;56:589–599.
Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217–223.
Anthony PP. Precursor lesions for liver cancer in humans. Cancer Res. 1976;36:2579–2583.
Rebello Pinto M, Koelma IA, Kumar D. Fine needle aspiration of focal liver lesions. Cytopathology 1994;5:359–368.
Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339.
Ferrell LD. Benign and Malignant Tumors of the Liver. In: Odze RD, Goldblum JR, Crawford R, eds. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia: Saunders; 2004:999–1014.
Ferrell L. Malignant liver tumors that mimic benign lesions: analysis of five distinct lesions. Semin Diagn Pathol. 1995;12:64–76.
Vogel UF, Bueltmann BD. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am J Clin Pathol. 2006;126:342–348.
Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981;75:734–738.
Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250.
Theodorsson-Norheim E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99.
Yasuda S, Arii S, Mori A, et al. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor 1 alpha and its significance. J Hepatol. 2004;40:117–123.
Majors BS, Betenbaugh MJ, Chiang GG. Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Metab Eng. 2007;9:317–326.
Kim W, Yoon JH, Jeong JM, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554–2562.
Shergill IS, Shergill NK, Arya M, Patel HR. Tissue microarrays: a current medical research tool. Curr Med Res Opin. 2004;20:707–712.
Jawhar NM. Tissue Microarray: a rapidly evolving diagnostic and research tool. Ann Saudi Med. 2009;29:123–127.
Hewitt SM. Tissue microarrays as a tool in the discovery and validation of predictive biomarkers. Methods Mol Biol. 2012;823:201–214.
Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES. Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol. 2003;16:79–84.
Riddle SR, Ahmad A, Ahmad S, et al. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol. 2000;278:L407–L416.
Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228.
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–364.
Gosmain Y, Lefai E, Ryser S, Roques M, Vidal H. Sterol regulatory element-binding protein-1 mediates the effect of insulin on hexokinase II gene expression in human muscle cells. Diabetes. 2004;53:321–329.
Kemmer N, Neff G, Secic M, Zacharias V, Kaiser T, Buell J. Ethnic differences in hepatocellular carcinoma: implications for liver transplantation. Dig Dis Sci. 2008;53:551–555.
Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939.
Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377.
Acknowledgments
This Project was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Sciences (CCTS) to G.G., N.H., and H.X. Award number ULRR029879 from the National Center for Research Resources, by Grants CA090764, AG025953 from the National Institutes of Health to N.H., and VA merit award BX000733 to N.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors recognize the contribution of the Research Histology and Tissue Imaging Core at the University of Illinois at Chicago to the completion of this Project (GG/gbg08232014).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzman, G., Chennuri, R., Chan, A. et al. Evidence for Heightened Hexokinase II Immunoexpression in Hepatocyte Dysplasia and Hepatocellular Carcinoma. Dig Dis Sci 60, 420–426 (2015). https://doi.org/10.1007/s10620-014-3364-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3364-3