Abstract
Background
The nervous system interacts dynamically with the immune system to modulate inflammation through humoral and neural pathways. However, the influence of visceral nerve (VN) on acute necrotizing pancreatitis (ANP) has drawn little attention.
Aim
To investigate the influence of VN on the pathophysiological process of ANP in dogs.
Methods
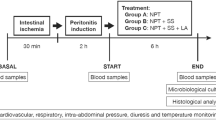
The dogs were divided into a sham operation (SO) group, ANP group, ANP + vagal nerve trunk transection (VNTT) group, and ANP + greater splanchnic nerve transection (GSNT) group. The VNTT and GSNT groups underwent VNTT and GSNT respectively immediately after ANP induction. The levels of serum pancreatic amylase (AMY), calcium, high-sensitivity C-reactive protein (HCRP), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-10 (IL-10) were monitored dynamically and the pathological examinations of the pancreas was performed at postoperative day 7.
Results
All serum parameters among the four groups showed no differences before the experiment (p > 0.05). At different postoperative times, the serum TNF-α, IL-1β, HCRP, and AMY were significantly increased, however, the serum calcium and IL-10 had dropped in the ANP group versus SO group (p < 0.05); an alike variation trend occurred between the VNTT group and ANP group (p < 0.05); an opposite variation trend occurred between the GSNT group and the ANP group (p < 0.05). The pancreas pathological scoring of VNTT group was highest in the four groups (p < 0.05) and GSNT group was lower versus ANP group (p < 0.05).
Conclusions
The GSNT has been shown to alleviate development of ANP, however, VNTT may exacerbate the ANP.




Similar content being viewed by others
References
Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Int Med. 2009;265:663–679.
Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859.
Pavlov VA, Parrish WR, Rosas-Ballina M, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45.
Kalsner S. Autoregulation of neurotransmitter release at autonomic nerve terminals: a questionable theory. J Auton Pharmacol. 2000;20:271–279.
Grogan EL, Morris JA Jr, Norris PR, et al. Reduced heart rate volatility: an early predictor of death in trauma patients. Ann Surg. 2004;240:547–556.
Wu C, Li Z. Mutt main pancreatic duct anatomy and pancreatitis model making. World Chin J Digestol. 1999;7:62–63.
Rongione AR, Kusske AM, Kan K, et al. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–967.
Steinman L. Elaborate interactions between immune and nervous systems. Nat Immunol. 2004;5:575–581.
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462.
Miksa M, Wu R, Zhou M, et al. Sympathetic excitotoxicity in sepsis: pro-inflammatory priming of macrophages by norepinephrine. Front Biosci. 2005;10:2217–2229.
Li J, Xiong J, Chen D, et al. Effect of vagotomy on acute pancreatitis in rats. J Tongji Med Univ. 2000;20:235–238.
Szklarczyk J, Jaworek J, Czech U, et al. Bilateral vagotomy attenuates the severity of secretagogue-induced acute pancreatitis in the rat. Adv Med Sci. 2014;39:1–6.
Carroll JK, Herrick B, Gipson T, et al. Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician. 2007;75:1513–1520.
Guice KS, Oldham KT, Remick DG, et al. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. J Surg Res. 1991;51:495–499.
Elfar M, Gaber LW, Sabek O, et al. The inflammatory cascade in acute pancreatitis: relevance to clinical disease. Surg Clin North Am, 2007;87:1325–1340, vii.
Zhang XP, Zhang J, Ma ML, et al. Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:83–87.
Xu P, Zhou XJ, Chen LQ, et al. Pioglitazone attenuates the severity of sodium taurocholate-induced severe acute pancreatitis. World J Gastroenterol. 2007;13:1983–1988.
The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228.
Demirag A, Pastor CM, Morel P, et al. Epidural anaesthesia restores pancreatic microcirculation and decreases the severity of acute pancreatitis. World J Gastroenterol. 2006;12:915–920.
Bernhardt A, Kortgen A, Niesel HCh, et al. Using epidural anesthesia in patients with acute pancreatitis–prospective study of 121 patients. Anaesthesiol Reanim. 2002;27:16–22.
Sun Jun–Jun, Chu Zhi-Jie, Liu Wei-Feng, et al. Perirenal space blocking restores gastrointestinal function in patients with severe acute pancreatitis. World J Gastroenterol. 2013;19:8752–8757.
Fritz S, Hackert T, Hartwig W, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200:111–117.
Sielenkamper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000;93:844–851.
Sielenkamper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000;93:844–851.
Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–642.
Leindler L, Morschl E, László F, et al. Importance of cytokines, nitric oxide, and apoptosis in the pathological process of necrotizing pancreatitis in rats. Pancreas. 2004;29:157–161.
Gerard C, Bruyns C, Marchant A, et al. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550.
Van Laethem JL, Eskinazi R, Louis H, et al. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut. 1998;43:408–413.
Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002;34:167–176.
Pezzilli R, Melzi D’eril GV, Morselli-labate AM, et al. Serum amyloid A, procalcitonin, and C-reactive protein in early assessment of acute pancreatitis. Dig Dis Sci. 2000;45:1072–1078.
Zhen G, Sun J, Liu W, et al. A1-acid glycoprotein and allergic C-reactive protein in the early acute pancreatitis severity evaluation of the dynamic monitoring of significance. Chin J Exp Surg. 2010;27:23–25.
Kylanpaa-Back ML, Takala A, Kemppainen E, et al. Procalcitonin strip teat in the early detection of severe acute pancreatitis. Br J Surg. 2001;88:222–227.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Jianqiang Mi in histopathological experiments. This study was funded by Cultivating Fund of Henan University of Science and Technology.
Conflict of interest
There are no potential conflicts of interest to disclose for all authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, JJ., Chu, ZJ., Zhang, YM. et al. Beneficial Effect of Splanchnic Nerve Transection and Harmful Effect of Vagotomy on Acute Necrotizing Pancreatitis in the Dog. Dig Dis Sci 60, 118–126 (2015). https://doi.org/10.1007/s10620-014-3315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3315-z