Abstract
Background
The autonomic nervous system can modulate the innate immune responses to bacterial infections via the splanchnic sympathetic nerves. Here, we aimed to determine the effects of bilateral splanchnic sympathetic nerve denervation on blood pressure, plasma cytokines, blood bacterial counts and the clinical state in sheep with established bacteremia.
Methods
Conscious Merino ewes received an intravenous infusion of Escherichia coli for 30 h (1 × 109 colony forming units/mL/h) to induce bacteremia. At 24 h, sheep were randomized to have bilaterally surgically implanted snares pulled to induce splanchnic denervation (N = 10), or not pulled (sham; N = 9).
Results
Splanchnic denervation did not affect mean arterial pressure (84 ± 3 vs. 84 ± 4 mmHg, mean ± SEM; PGroup = 0.7) compared with sham treatment at 30-h of bacteremia. Splanchnic denervation increased the plasma levels of the pro-inflammatory cytokine interleukin-6 (9.2 ± 2.5 vs. 3.8 ± 0.3 ng/mL, PGroup = 0.031) at 25-h and reduced blood bacterial counts (2.31 ± 0.45 vs. 3.45 ± 0.11 log10 [CFU/mL + 1], PGroup = 0.027) at 26-h compared with sham treatment. Plasma interleukin-6 and blood bacterial counts returned to sham levels by 30-h. There were no differences in the number of bacteria present within the liver (PGroup = 0.3). However, there was a sustained improvement in clinical status, characterized by reduced respiratory rate (PGroup = 0.024) and increased cumulative water consumption (PGroup = 0.008) in splanchnic denervation compared with sham treatment.
Conclusion
In experimental Gram-negative bacteremia, interrupting splanchnic sympathetic nerve activity increased plasma interleukin-6, accelerated bacterial clearance, and improved clinical state without inducing hypotension. These findings suggest that splanchnic neural manipulation is a potential target for pharmacological or non-pharmacological interventions.
Similar content being viewed by others
Background
A dysregulated immune response to bacterial infections can be a common trigger for sepsis [1, 2]. Reductions in blood pressure in response to systemic bacterial infections elicit a baroreflex-mediated increase in sympathetic nerve activity (SNA) [3, 4], which would be expected to promote peripheral vasoconstriction. However, this effect appears offset by the profoundly elevated release of vasodilators such as nitric oxide, leading to a net reduction in total peripheral conductance and mean arterial pressure (MAP). There is, however, accumulating evidence that protracted overactivation of the splanchnic sympathetic nerves can exacerbate the sepsis-associated immune suppression and promote bacterial growth [5,6,7]. Accordingly, developing interventions to modulate splanchnic SNA may provide a new therapeutic avenue for sepsis.
In response to systemic inflammation, we established that the brain activates a powerful anti-inflammatory reflex through the splanchnic sympathetic nerves [8]. In a rodent model of endotoxemia, we demonstrated that an anti-inflammatory reflex mediated by the splanchnic sympathetic nerves strongly suppresses tumor necrosis factor alpha (TNF-α) response, within 1.5-h of lipopolysaccharide treatment [8, 9]. In addition, we showed that sheep with previously cut splanchnic nerves (to disable the reflex) had an enhanced TNF-α response within 1.5-h and interleukin-6 (IL-6) response within 6-h of an intravenous bolus of live Escherichia coli (E. coli). Such animals also cleared their bacteremia more rapidly [10]. However, implementing interventions prior to the onset of bacteremia lacks clinical translatability, because most patients treated in intensive care units have established infections [11, 12]. Additionally, the safety of denervating a major resistance vascular bed such as the splanchnic circulation in the late stages of bacterial infection remains unknown.
Accordingly, we aimed to assess the safety and effectiveness of splanchnic denervation after 24-h of a systemic Gram-negative infection in a well-validated sheep model [13]. We investigated whether splanchnic denervation was able to reverse immunosuppression, accelerate bacterial clearance and improve the clinical state when it was applied during established bacteremia rather than beforehand. As the liver is established as a vital organ involved in limiting the systemic dissemination of bacteria in response to infection [14], we also assessed whether splanchnic denervation accelerated the number of E. coli sequestrated in the liver. We tested the hypothesis that splanchnic sympathetic nerve denervation would increase plasma levels of proinflammatory cytokines, accelerate bacterial clearance, increase liver sequestration, and thereby aid clinical recovery in sheep with established bacteremia.
Materials and methods
Animal preparation
The experiments were performed in accordance with the National Health and Medical Research Council of Australia guidelines and approved by the Animal Ethics Committee of the Florey Institute of Neuroscience and Mental Health [Ethics ID: 20-040-FINMH]. All studies were performed in accordance with the Animal Research: Reporting of In Vivo Experiments (arrive) criteria [15]. Merino Ewes (35–45 kg) aged between 1.5–2.5 years were housed in individual metabolic cages with access to 800 g of oaten chaff and 6.5 L of water daily.
Surgical preparation
Sheep underwent a preparatory surgical procedure under sterile conditions. General anesthesia was induced with 15 mg/kg of sodium thiopental (intravenous, [i.v.], Jurox Pty Limited, NSW, Australia) and following tracheal intubation, maintained on 2.0 – 2.5% isoflurane (Pharmachem, QLD, Australia) at an inspired oxygen fraction of 60%.
The splanchnic sympathetic nerves were bilaterally exposed retroperitoneally via flank incisions. Once a splanchnic sympathetic nerve was isolated, a silk snare was passed under each nerve (length 30 cm, size 3-O; Dynek PTY LTD, SA, Australia). Both ends of the snare were passed up a vinyl tube (length 20 cm, ID 1.5 mm, OD 2.5 mm; Dural Plastics & Engineering, NSW, Australia) and a polyethylene cannula (length 25 cm, ID 0.58 mm, OD 0.96 mm; Microtube Extrusions, NSW, Australia) was pushed down the vinyl tube so that its tip was adjacent to the nerve for the administration of an anesthetic agent prior to denervating the animals in the treatment group (Additional file 1: Fig. S1). An in-house fabricated thermocouple was implanted in the retroperitoneal space to measure core body temperature. The tubes encasing the snare and the cannula were tunneled subcutaneously and exteriorized on the back of the sheep.
Two polyethene cannulae were inserted into the right jugular vein for the administration of E. coli (ID 0.58 mm, OD 0.96 mm; Microtube Extrusions, NSW, Australia) and maintenance fluid infusions (ID 1.18 mm, OD 1.7 mm; Microtube Extrusions, NSW, Australia). A tygon cannula (ID 1.0 mm, OD 1.8 mm; Cole-Parmer, VIC, Australia) was inserted into the right carotid artery for blood sampling and measurement of MAP and heart rate (HR).
Animals were administered intramuscular antibiotics (penicillin, 900 mg Ilium, Troy Laboratories, VIC, Australia) under general anesthesia and then at 24- and 48-h postoperatively. Flunixin meglumine (50 mg, intramuscular; Flunixon, Norbrook, Tullamarine, Australia) was administered at surgery and then 4-h postoperatively for analgesia. Animals were allowed a 3-day recovery period before initiating the experimental protocol.
Experimental protocol
Induction of Gram-negative bacteremia
After a 20-h baseline period, bacteremia was induced by continuous intravenous infusion of E. coli for 30-h (1 X 109 colony forming units [CFU]/mL, 0.6 mL/h). This dose of E. coli has been previously reported to induce a clinically relevant hemodynamic profile in Gram-negative bacteremia in sheep [18, 19]. Sheep were given a maintenance infusion of Hartmann’s solution (i.v., 2 mL/kg/h; Baxter Pharmaceutical Solutions LLC, NSW, Australia) from the end of the baseline period to the end of the experiment, which was limited to 30-h of bacteremia before the animal was euthanized (Fig. 1).
Experimental timeline from baseline to 30-h of Gram-negative bacteremia. Timepoint − 20 represents the 20 h of baseline recordings. Timepoint 0 indicates the 20th h of baseline recordings prior to starting the continuous infusion of E. coli. All animals were given a maintenance fluid infusion of Hartmann’s solution (-20 to 30-h from inducing bacteremia) and a continuous infusion of E. coli (0 to 30-h from inducing bacteremia). At 24-h of bacteremia animals underwent either splanchnic denervation or sham procedure. In bold are the sampling timepoints (0, 23, 24, 25, 27 and 30 from inducing Gram-negative bacteremia)
Intervention
At 24-h of bacteraemia, conscious sheep were randomly allocated to either the splanchnic-denervated (n = 10) or sham-denervated (sham n = 9) group. Sheep allocated to the splanchnic-denervated group were given 4 mL of lignocaine (20 mg/mL, ilium, Troy Laboratories PTY Limited, NSW, Australia) via the implanted polyethylene cannula, to avert pain when the snares implanted on the right-flank were pulled. Five minutes later, the splanchnic nerve snares were bilaterally pulled and clamped. After 5-min, this splanchnic denervation process was repeated contralaterally on the left flank of the animals. Sheep allocated to the sham group were given 4 mL of saline and were left untreated for a similar period. There was no blood sampling during 24–25 h in both groups. In both groups the bacterial infusion was continued for a further 6 h (total 30-h), after which the animals were euthanized (pentobarbitone sodium, 100 mg/kg, i.v., Vibrac PTY Limited, NSW, Australia). At post-mortem, the effectiveness of splanchnic denervation was confirmed, and sections of the liver were collected for immunohistochemical analysis of E. coli localization (Fig. 1).
Sampling and recording
Arterial blood samples were collected at the 20th h of baseline, 23rd h of bacteremia (before splanchnic-denervation or sham procedure) and then after intervention at the 25th, 27th and 30th h of bacteremia. Cumulative food and water consumption and respiratory rate were measured during the 20th h of baseline, at 23-h of bacteremia and then at hourly intervals after splanchnic-denervation or sham treatment interventions (from 24-to-30-h of bacteremia) to assess clinical state. MAP, HR, and core body temperature were continuously recorded at 100 Hz on a computer using a CED micro 1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge, United Kingdom).
Immunohistochemical analysis
At post-mortem, sections of liver (approximately 5–6 g) were collected and fixed in 10% neutral buffered formalin. Samples were then dehydrated, paraffin embedded and 5 µm slices slide mounted, as previously described (Histology Core Services, Florey Institute) [16]. Slides were deparaffinized using xylene, cleared in graded alcohols, and rehydrated in 0.1 M PBS. Sections were stained using a rabbit anti-E. coli primary antibody (Abcam, Cambridge, United Kingdom; 1:500) at room temperature overnight. After washes, sections were then incubated in a Biotin-tagged anti-rabbit antibody (Jackson ImmunoResearch; 1:500) for 1-h at room temperature followed by Streptavidin-Alexa Fluor®488 conjugated secondary antibody (Jackson ImmunoResearch; 1:500) for 1-h at room temperature. Tissues were coverslipped with mounting medium containing DAPI (Sigma-Aldrich). Images were obtained using a Leica DMI6000 inverted confocal microscope at × 20 magnification. For each liver section six images were taken: three around hepatic vessels and three in the liver parenchyma between major vessels. E. coli were viewed using the Leica LasX software and then counted manually. The investigators undertaking the E. coli counting were blinded to the treatment groups.
Cytokine analysis
Arterial plasma levels of IL-6, IL-8, IL-10, interferon-gamma (IFN-γ), and TNF-α were analysed using manufacturer validated enzyme-linked immunosorbent ovine assays (ELISA) (Kingfisher Biotech Inc., MN, USA) in accordance with the manufacturer’s guidelines (refer to Additional file 1: Table S1), as previously described [10].
All ELISA kits followed the same protocol, incubation with the capture antibody (2.5 µg/mL) and blocking buffer (4% BSA in DPBS). Arterial blood samples were collected in 5 mL EDTA tubes (Sarstedt, Germany), centrifuged at 3000 g and the aliquoted plasma was stored at − 80 ℃ for subsequent analysis. ELISA kits included the standard curve reagents (25 ng/mL top standard was serially diluted 1:1 to create the standard curve), the detection antibody (0.4 µg/mL), Streptavidin-HRP (Kingfisher Biotech Inc., MN, USA, catalogue number AR0068-001), 3,3’,5,5’-tetramethylbenzidine substrate solution (Kingfisher Biotech Inc., MN, USA, catalogue number AR0133-002) and a 0.18 M Sulfuric Acid stop solution. All plates were read at 450 nm (CLARIOstarPlus, BMG LABTECH, Australia). Samples were run in duplicates, and a coefficient of variability under 10% was classified as acceptable. No samples were re-run because they all fell within range of the standard curve.
Bacterial blood count analysis
To quantify E. coli levels in the blood, a colony forming assay was used, as previously described [10]. Blood samples were serially diluted 1 in 10 in sterile 0.01 M PBS; 20 μL of undiluted and diluted blood were spotted onto Luria–Bertani agar plates and incubated in air overnight at 37 °C. Colonies were counted and expressed as CFU/mL.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) after passing the tests for normality (D’Agostino and Pearson Omnibus test, confirmed by Kolmogorov–Smirnov test). Specific time-point comparisons within each group (splanchnic-denervated or sham) were performed using a paired Student t-tests with a Bonferroni correction of K = 2 to assess differences between baseline and 23-h and 30-h of bacteremia. An unpaired Student t-test was used to compare the E. coli (count) present between the sham and splanchnic-denervated group in the hepatic parenchyma and around hepatic vessels. A two-way repeated measures analysis of variance (ANOVA) was used to assess differences of MAP, HR, core body temperature, cytokines, bacterial counts, blood biochemistry, water consumption, food consumption and respiratory rate between splanchnic-denervated vs sham (Group), Time (from 24 to 30-h of bacteremia) and their interaction (GroupxTime). To correct for multiple comparisons a Šidák test was utilized (GraphPad Prism 8.0; GraphPad Software, La Jolla, CA). A two-sided P value < 0.05 was considered to be significant.
Results
Cardiovascular hemodynamics and febrile reponse to bacteremia and splanchnic denervation
There were no significant differences between the groups in the cardiovascular variables and body temperature over the 20-h baseline period.
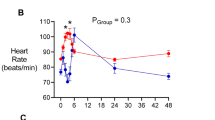
In the sham group, MAP progressively declined from pre-morbid baseline levels until 30-h of bacteremia, but this overt effect was not statistically significant after adjusting for multiple comparisons (95 ± 3 to 84 ± 4 mmHg, P = 0.064) (unadjusted P = 0.032). There were no significant differences in the magnitude of the reduction in MAP between sham and splanchnic-denervated groups (Fig. 2A). Bacteremia was further characterized by increases in HR (89 ± 4 to 137 ± 7 beats/min, P < 0.001) and core body temperature (39.7 ± 0.2 to 41.0 ± 0.3 °C, P = 0.024) in the sham group by 30-h of E. coli infusion. The degree of tachycardia and increase in core temperature were not significantly different between treatment groups (Fig. 2B, C). The bacteremia-induced changes in arterial biochemistry were similar between the groups from 24-to-30-h of bacteremia (Table 1).
Changes in systemic hemodynamic in response to bilateral splanchnic denervation or sham procedure during ovine Gram-negative bacteremia. Mean arterial pressure (A), heart rate (B), and core body temperature (C) during infusion of Escherichia coli from 0 to 30-h. Animals were randomized to either splanchnic-denervated (n = 10) or untreated (sham, n = 9) at 24-h of bacteremia. Baseline is the mean of the 20th hour of the baseline period, and times 23 to 30-h of bacteremia are means of 1-h intervals. Data are presented as treatment group-specific mean ± SEM. P values represent the results of a 2-way ANOVA assessing differences between the groups (splanchnic-denervated vs. sham) and treatment time points (from 24 to 30-h of Gram-negative bacteremia) and the interaction between group and time. A two-sided P value < 0.05 was considered to be significant
Pro- and anti-inflammatory cytokine responses to bacteremia and splanchnic denervation
At 23-h of bacteremia, prior to splanchnic denervation, there were similar increases in the plasma concentrations of the pro-inflammatory cytokine, IL-6, in the sham (from 2.84 ± 0.23 to 4.42 ± 0.706 ng/mL, P = 0.049) and intervention groups, (3.46 ± 0.74 to 5.20 ± 0.76 ng/mL, P = 0.027). From 25-to-30-h of bacteremia, IL-6 levels remained consistently elevated in the sham group. In contrast, there was a further, transient elevation in plasma IL-6 levels following bilateral splanchnic denervation at 25-h of bacteremia (to 9.23 ± 2.54 ng/mL, PGroup = 0.031), before declining to levels that were similar to those in the sham treatment group by 30-h of bacteremia (to 6.26 ± 1.55 ng/mL) (Fig. 3A).
Changes in plasma inflammatory and anti-inflammatory cytokines in response to bilateral splanchnic denervation or sham procedure during ovine Gram-negative bacteremia. Plasma IL-6 (A), IFN-γ (B), IL-8 (C), IL-10 (D) and TNF-α (E) during infusion of E. coli from 0 to 30-h of bacteremia. Animals were randomized to either splanchnic-denervated (n = 9) or untreated (sham, n = 9) at 24-h of bacteremia. Baseline, 23-h, 25-h, 27-h and 30-h are the sampled timepoints. Data are presented as treatment group-specific mean ± SEM. P values represent the results of a 2-way ANOVA assessing differences between the groups (splanchnic-denervated vs sham) and treatment time points (from 24 to 30-h of bacteremia) and the interaction between group and time. A two-sided P value < 0.05 was considered to be significant
At 23-h of bacteremia, there was also an increase in the plasma concentrations of the anti-inflammatory cytokine, IL-10, in both the sham (from 0.44 ± 0.17 to 1.72 ± 0.51 ng/mL, P = 0.014) and splanchnic-denervated groups (from 0.50 ± 0.14 to 1.56 ± 0.36 ng/mL, P = 0.033) (Fig. 3D). These plasma concentrations of IL-10 remained similarly elevated in the sham and splanchnic-denervated groups from 25-to-30-h of bacteremia (Fig. 3D). There were also no significant differences between the sham and splanchnic-denervated groups in plasma concentrations of TNF-α, IL-8 and IFN-γ by 23-h of bacteremia or after intervention from 24-to-30-h (Fig. 3B, C, E).
Effects of bacteremia and splanchnic denervation on blood bacterial counts
There were significant elevations in blood E. coli counts from undetectable levels at baseline to 23-h of bacteremia in the sham (to 3.41 ± 0.16 log10[CFU/mL + 1], P < 0.001) and splanchnic denervation groups (to 3.27 ± 0.15 log10[CFU/mL + 1], P < 0.001), prior to treatment allocation (Fig. 4). Blood bacterial counts remained elevated in the sham group in response to a continuous infusion of E. coli for 30-h (to 2.99 ± 0.51 log10[CFU/mL + 1]). In contrast, there was a transient reduction in blood E. coli counts in the splanchnic-denervated group by 26-h of bacteremia (2.31 ± 0.45 log10[CFU/mL + 1]; PGroup = 0.024), before returning to sham treatment levels by 30-h of E. coli infusion (to 2.82 ± 0.36 log10[CFU/mL + 1]; Fig. 4).
Changes in blood bacterial counts in response to bilateral splanchnic denervation or sham procedure during ovine Gram-negative bacteremia. Animals were randomized to either splanchnic-denervated (n = 9) or untreated (sham, n = 7) at 24-h of bacteremia. Baseline, 23-h, 25-h, 26-h, 27-h and 30-h are the sampling timepoints. Data are presented as treatment group-specific mean ± SEM. P values represent the results of a 2-way ANOVA assessing differences between the groups (splanchnic-denervated vs. sham) and treatment time points (from 24 to 30-h of bacteremia) and the interaction between group and time. A two-sided P value < 0.05 was considered to be significant
Effects of bacteremia and splanchnic denervation on clinical state
Splanchnic denervation-associated improvements in clinical state were accompanied by significant elevations in cumulative water intake from 24 to 30-h of bacteraemia (PGroup = 0.008). Cumulative food intake also showed a tendency to be higher in splanchnic-denervated sheep compared with sham treatment, but this overt effect did not reach statistical significance (PGroup = 0.063) (Fig. 5A, C). Respiratory rate was elevated by 23-h of bacteraemia in both groups prior to splanchnic denervation (18 ± 1 to 37 ± 4 breaths/min; P = 0.001) or sham treatment (19 ± 1 to 38 ± 5 breaths/min; P = 0.008) compared with pre-morbid baseline levels. The degree of tachypnoea was significantly reduced following splanchnic denervation (to 23 ± 1 breaths/min) compared with sham treatment (to 43 ± 4 breaths/min) by 30-h of bacteraemia (PGroup = 0.024) (Fig. 5B).
Changes in clinical status in response to bilateral splanchnic denervation or sham during ovine Gram-negative bacteremia. Cumulative food consumption (A), respiration rate (B) and cumulative water consumption (C), during infusion of Escherichia coli from 0 to 30-h. Animals were randomized to either splanchnic-denervated (n = 10) or untreated (sham, n = 9) at 24-h of bacteremia. Baseline is the average of the 20th hour of the baseline period, and times 23 to 30-h of bacteremia are means of 1-h intervals. Data are presented as treatment group-specific mean ± SEM. P values represent the results of a 2-way ANOVA assessing differences between the groups (splanchnic-denervated vs. sham) and treatment time points (from 24 to 30-h of Gram-negative bacteremia) and the interaction between group and time. A two-sided P value < 0.05 was considered to be significant
Effect of splanchnic denervation on the localization of E. coli in the liver
E. coli were found within the liver parenchyma of both experimental groups at post-mortem after 30-h of bacteremia. There were no significant differences between the sham and splanchnic-denervated groups in the numbers of E. coli present within the hepatic parenchyma (52.7 ± 16.1 vs 39.1 ± 3.7; P = 0.3) and around the hepatic vessels (45.3 ± 4.9 vs 38.6 ± 5.3; P = 0.4) (Fig. 6).
Counts of E. coli in the liver of sham and splanchnic-denervated sheep after 30-h of bacteremia. A Numbers of E. coli in hepatic parenchyma or around hepatic vessels in sham and splanchnic-denervated animals (n/group; NS two-tailed unpaired Student T test). B Representative image of hepatic parenchyma surrounding hepatic vessels (HV) taken at × 40 magnification showing the localization of E. coli (green) and cell nuclei are stained with DAPI (blue). White solid arrows indicate E. coli and white dashed arrows indicate the cell nuclei. Scale bar 50 µm
Discussion
Key findings
In a large mammalian model of live Gram-negative bacteremia, we determined the effect of bilaterally denervating the splanchnic sympathetic nerves, at a clinically appropriate interventional time-point of established infection. We found that splanchnic denervation transiently increased plasma levels of the pro-inflammatory cytokine, IL-6, and this was followed by a transient improvement in bacterial clearance and a sustained improvement in cumulative water intake and respiratory rate. Moreover, we demonstrated that splanchnic denervation did not exacerbate bacteremia-induced reductions in blood pressure. Finally, we found that this intervention did not change the level of sequestration of bacteria in the liver.
Relationship to previous studies
In sepsis, a protracted sympathetic overstimulation has been proposed to contribute to the decreased vasopressor responsiveness to endogenous catecholamines and exogenously infused vasopressor agents, leading to severe hypotension and reduced organ perfusion [17]. Supporting this notion, we have previously reported that centrally acting sympatholytics, dexmedetomidine and clonidine, improve blood pressure management and sensitivity to catecholamine and non-catecholamine vasopressors (noradrenaline, phenylephrine, and angiotensin II) in established ovine Gram-negative sepsis [18, 19]. Similarly, clinical studies in human sepsis have reported that treatment with dexmedetomidine results in lower vasopressor requirements to maintain target MAP [20, 21]. These studies indicate that sympatho-modulatory interventions can confer a beneficial role on cardiovascular function in established infections. However, the use of non-selective sympatholytic drugs including clonidine and dexmedetomidine attenuated the increase in plasma IL-6 and enhanced the increase in IL-10 levels in ovine sepsis, likely due to activation of anti-inflammatory cholinergic pathways [19, 22]. Collectively, these studies provided the rationale to selectively target the splanchnic sympathetic nerves to demonstrate the safety and effectiveness of such an intervention in a non-hypotensive ovine model of bacteremia.
The splanchnic sympathetic nerves are implicated as the efferent arm of a negative feedback mechanism whereby the brain inhibits innate immune responses to inflammatory stimuli [8,9,10, 23,24,25,26,27,28]. We previously reported that in rats made endotoxemic with intravenous lipopolysaccharide (LPS), prior section of the splanchnic sympathetic nerves resulted in enhanced plasma levels of pro-inflammatory cytokines (TNF-α, IL-6 and IFN-γ) and a reduction in the anti-inflammatory cytokine, IL-10 [27]. Subsequently we reported that sheep with previously sectioned splanchnic nerves mounted an exaggerated inflammatory cytokine response to an intravenous bolus injection of live E. coli and rapidly cleared the bacteria from their bloodstream [10].
Cytokine responses
In the current study, we show that, sectioning the splanchnic nerves during established Gram-negative bacteraemia, enhances IL-6 levels, albeit transiently. These responses demonstrate a positive biological signal of splanchnic denervation when compared with our previous findings that plasma IL-6 levels temporally declined from 24 to 32-h of sepsis in sheep with established hypotensive sepsis [19, 29, 30]. Moreover, these enhanced IL-6 responses were followed by accelerated bacterial clearance and improved clinical state, despite no treatment with antibiotics. IL-6 is an important contributor to the pro-inflammatory response to infections that remains elevated up to 32-h of sepsis [31, 32]. This contrasts with plasma levels of other inflammatory cytokines including TNF-α and IFN-γ that rise transiently in early stages of infection and then fall towards baseline levels at later stages of sepsis [10, 33, 34]. It is therefore not surprising that we observed no detectable change in plasma TNF-α and IFN- γ by 23-h of bacteraemia, nor any response of those cytokines to splanchnic nerve denervation.
Bacterial clearance
The transient elevation in IL-6 induced by splanchnic denervation was followed by an expedited clearance of bacteria from the bloodstream until 3-h post splanchnic denervation. Monocytes, macrophages, and neutrophils contain IL-6 receptors, and their function is regulated by IL-6 signaling pathways [32]. At the onset of the innate immune response to a pathogen entering the bloodstream, neutrophils are the primary phagocytes involved in limiting the systemic dissemination of the infection. IL-6 plays an important role in modulating the magnitude of the neutrophil response [35, 36]. As the inflammatory response continues, monocytes and macrophages are recruited to assist [31] and IL-6 is an important regulator in this transition, ensuring that inflammation is sufficient to continue mounting an adequate response to a persisting infection [35, 36]. In the current study, the elevated IL-6 levels after splanchnic-nerve denervation may have stimulated these innate immune cells to expedite the clearance of E coli from the blood. The inability to assess the direct interaction between IL-6 and neutrophil function is a limitation of the current experimental design that warrants further investigation following either non-pharmacological or pharmacological inhibition of the splanchnic sympathetic nerves in future experiments.
Interestingly, splanchnic denervation at 24-h of bacteremia did not significantly affect IL-10 levels. Differential regulation of IL-6 and IL-10 responses to splanchnic nerve denervation aligns with our recent findings in rodent endotoxemia. We recently found that distinct splanchnic sympathetic efferent pathways regulate pro- and anti-inflammatory cytokines in response to inflammatory stimuli [37]. The neural-driven adrenaline, acting via β2 adrenoreceptors, regulates IL-10 responses. In contrast, the increase in TNF-α, which stimulates the subsequent IL-6 release in response to endotoxemia, is restrained by the splanchnic nerves that provide neural control to abdominal organs including the spleen and involves non-β2- adrenoreceptor mechanisms [37].
Clinical state
Following splanchnic sympathetic denervation, there was a sustained improvement in clinical status, despite blood bacterial counts returning to sham treatment levels by 30-h. The liver plays a vital role in the clearance of pathogens entering the bloodstream to mitigate the spread of bacteria to more susceptible organs including the brain and kidneys [14, 38, 39]. Accordingly, the similarities in blood bacterial counts by 30-h of infection were confirmed by our immunohistochemical analysis of the liver tissue, which demonstrated similar numbers of E. coli present in the hepatic parenchyma and hepatic vessels in both groups. The restoration of IL-6 and bacterial counts in denervated animals to sham levels by the end of the experiment (30-h) is likely due to the continued infusion of live E. coli outstripping the enhanced bacterial clearance. It is conceivable that the initiation in improvement in clinical state could be attributed to an enhanced immune response and expedited bacterial clearance, which were evident until 27-h of bacteraemia. It is also important to note that snaring the splanchnic nerves will destroy not only the efferent but also the afferent fibres that mediate visceral pain and febrile signalling induced by the bacteraemia [40,41,42]. This may have contributed to the sustained clinical improvement after splanchnic denervation, despite a rebound in bacterial counts. Future studies are therefore warranted to test the effect of splanchnic nerve section on blood pressure and bacterial clearance in more severe models of Gram-negative sepsis-induced hypotension, without further E. coli infusion and/or with antibiotic treatment, which may better mimic patients with sepsis after removal of the infection source in ICUs [43].
Conclusions
Our findings demonstrate that interrupting splanchnic nerve activity in established bacteremia is safe for blood pressure control, increases circulating IL-6 levels, expedites the clearance of bacteria from the bloodstream and improves the clinical state. Although the enhanced bacterial clearance was transit in the face of a continuous intravenous infusion of bacteria, such an intervention may prove more effective in cases where the infection source is removed. Importantly, our findings show that the neural reflex persists for at least 24-h into established bacteremia, and that its sustained anti-inflammatory action may hamper the ability of innate immune mechanisms to overcome an infection. Future studies are warranted to explore the effects of pharmacological and other means, such as acute nerve block [44], to inhibit splanchnic sympathetic nerve activity in the setting of severe bacterial infection.
Availability of data and materials
Data available upon request due to ethical reasons.
Abbreviations
- SNA:
-
Sympathetic nerve activity
- MAP:
-
Mean arterial pressure
- E. coli :
-
Escherichia coli
- i.v.:
-
Intravenous
- HR:
-
Heart rate
- CFU:
-
Colony forming units
- IL-6:
-
Interleukin-6
- IFN-γ:
-
Interferon-gamma
- IL-8:
-
Interleukin-8
- IL-10:
-
Interleukin-10
- TNF-α:
-
Tumour necrosis factor-alpha
- ELISA:
-
Enzyme-linked immunosorbent assay
- SEM:
-
Standard error of the mean
- ANOVA:
-
Analysis of variance
- LPS:
-
Lipopolysaccharide
References
Brun-Buisson C, Doyon F, Carlet J (1996) Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit 154(3):617–624
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Booth LC, Ramchandra R, Calzavacca P, May CN (2014) Role of prostaglandins in determining the increased cardiac sympathetic nerve activity in ovine sepsis. Am J Physiol Regul Integr Comp Physiol 307(1):R75–R81
May C, Frithiof R, Hood S, McAllen R, McKinley M, Ramchandra R (2010) Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp Physiol 95(1):34–40
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52(4):595–638
Freestone PP, Sandrini SM, Haigh RD, Lyte M (2008) Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol 16(2):55–64
McAllen RM, McKinley MJ, Martelli D (2022) Reflex regulation of systemic inflammation by the autonomic nervous system. Auton Neurosci 237:102926
Martelli D, Yao S, McKinley M, McAllen R (2014) Reflex control of inflammation by sympathetic nerves, not the vagus. Physiol J 592(7):1677–1686
Occhinegro A, Wong CY, Chua BY, Jackson DC, McKinley MJ, McAllen RM et al (2021) The endogenous inflammatory reflex inhibits the inflammatory response to different immune challenges in mice. Brain Behav Immun 97:371–375
Lankadeva YR, May CN, McKinley MJ, Neeland MR, Ma S, Hocking DM et al (2020) Sympathetic nerves control bacterial clearance. Sci Rep 10(1):1–8
Lelubre C, Vincent J-L (2018) Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol 14(7):417–427
Caraballo C, Jaimes F (2019) Organ dysfunction in sepsis: an ominous trajectory from infection to death. Yale J Biol Med 92(4):629–640
Lankadeva YR, Kosaka J, Evans RG, May CN (2018) An ovine model for studying the pathophysiology of septic acute kidney injury. Traum and Isch Inj. https://doi.org/10.1007/978-1-4939-7526-6_16
Zeng Z, Surewaard BG, Wong CH, Geoghegan JA, Jenne CN, Kubes P (2016) CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe 20(1):99–106
Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H (2007) Influence of poly I: C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun 21(4):477–489
McArdle Z, Pontes RB, Yao ST, Lankadeva YR, Singh RR, Hood SG et al (2019) Blunted diuretic and natriuretic responses to acute sodium loading early after catheter-based renal denervation in normotensive sheep. Am J Physiol Regul Integr Comp Physiol 317(2):R319–R327
Pichot C, Geloen A, Ghignone M, Quintin L (2010) Alpha-2 agonists to reduce vasopressor requirements in septic shock? Med Hypotheses 75(6):652–656
Lankadeva YR, Booth LC, Kosaka J, Evans RG, Quintin L, Bellomo R et al (2015) Clonidine restores pressor responsiveness to phenylephrine and angiotensin II in ovine sepsis. Crit Care Med 43(7):e221–e229
Lankadeva YR, Ma S, Iguchi N, Evans RG, Hood SG, Farmer DG et al (2019) Dexmedetomidine reduces norepinephrine requirements and preserves renal oxygenation and function in ovine septic acute kidney injury. Kidney Int 96(5):1150–1161
Cioccari L, Luethi N, Bailey M, Shehabi Y, Howe B, Messmer AS et al (2020) The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: a subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit Care 24(1):1–13
Morelli A, Sanfilippo F, Arnemann P, Hessler M, Kampmeier TG, D’Egidio A et al (2019) The effect of propofol and dexmedetomidine sedation on norepinephrine requirements in septic shock patients: a crossover trial. Crit Care Med 47(2):e89–e95. https://doi.org/10.1097/ccm.0000000000003520
Calzavacca P, Booth LC, Lankadeva YR, Bailey SR, Burrell LM, Bailey M et al (2019) Effects of clonidine on the cardiovascular, renal, and inflammatory responses to experimental bacteremia. Shock 51(3):348–355
Bratton B, Martelli D, McKinley M, Trevaks D, Anderson C, McAllen R (2012) Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol 97(11):1180–1185
Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM, Martelli D (2018) Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun 73:441–449
Martelli D, Farmer D, Yao S (2016) The splanchnic anti-inflammatory pathway: could it be the efferent arm of the inflammatory reflex? Exp Physiol 101(10):1245–1252
Martelli D, Farmer DG, McKinley MJ, Yao ST, McAllen RM (2019) Anti-inflammatory reflex action of splanchnic sympathetic nerves is distributed across abdominal organs. Am J Physiol Regul Integr Comp Physiol 316(3):R235–R242
Martelli D, Yao ST, Mancera J, McKinley MJ, McAllen RM (2014) Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anesthesia. Am J Physiol Regul Integr Comp Physiol 307(9):R1085–R1091
Martelli D, Yao ST, McKinley MJ, McAllen RM (2014) Neural control of inflammation by the greater splanchnic nerves. Temperature 1(1):14–15
Lankadeva YR, Kosaka J, Evans RG, Bellomo R, May CN (2018) Urinary oxygenation as a surrogate measure of medullary oxygenation during angiotensin II therapy in septic acute kidney injury. Crit Care Med 46(1):e41–e48
Lankadeva YR, Kosaka J, Evans RG, Bailey SR, Bellomo R, May CN (2016) Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int 90(1):100–108
Chousterman BG, Swirski FK, Weber GF (2017) Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39:517–528
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem 374(1):1–20
Ono S, Ueno C, Aosasa S, Tsujimoto H, Seki S, Mochizuki H (2001) Severe sepsis induces deficient interferon-gamma and interleukin-12 production, but interleukin-12 therapy improves survival in peritonitis. Am J Surg 182(5):491–497
Kohler J, Heumann D, Garotta G, LeRoy D, Bailat S, Barras C et al (1993) IFN-gamma involvement in the severity of gram-negative infections in mice. J Immunol 151(2):916–921
Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N et al (2001) Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14(6):705–714
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F et al (1998) IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Inves 101(2):311–320
McKinley MJ, Martelli D, Trevizan-Baú P, McAllen RM (2022) Divergent splanchnic sympathetic efferent nerve pathways regulate IL-10 and TNF responses to endotoxaemia. Physiol J. https://doi.org/10.1113/JP283217
Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS (2001) Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 281(4):L913–L921
Dhainaut J-F, Marin N, Mignon A, Vinsonneau C (2001) Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 29(7):S42–S47
Romanovsky AA (2004) Signaling the brain in the early sickness syndrome: are sensory nerves involved? Front Biosci Landmark 9(1):494–504
Grundy D (1988) Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J Auton Nerv Syst 22(3):175–180
Ozaki N, Gebhart G (2001) Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest 281(6):G1449–G1459
Dugar S, Choudhary C, Duggal A (2020) Sepsis and septic shock: guideline-based management. Cleve Clin J Med 87(1):53–64
Fudim M, Jones WS, Boortz-Marx RL, Ganesh A, Green CL, Hernandez AF et al (2018) Splanchnic nerve block for acute heart failure. Circulation 138(9):951–953
Acknowledgements
The authors would like to thank Mr. Tony Dorman, Mr. Tom Vale, and Mr. Quan Nguyen for their excellent technical assistance. We would like to thank Ms. Melinda Goga for assisting with the plasma cytokine analysis. We would like to thank Dr. Ian Birchall for performing the paraffin blocking, slicing, and mounting the sections of liver for immunohistochemical analysis. Lastly, we would like to thank Dr. Dianna Hocking for mentoring the researchers in performing blood bacterial counts.
Funding
This study was funded by the National Health and Medical Research Council of Australia [GNT1186382]. YRL was funded by a Future Leader Fellowship from the National Heart Foundation of Australia [FLF105666].
Author information
Authors and Affiliations
Contributions
YRL, CNM, LCB, RMM, MJM, DM, RB: conception or design of the surgical and experimental protocols. RMP, LB, SH: acquisition, analysis, and interpretation of data. YRL, RMP, CNM, RMM: drafting of the manuscript. YRL, RMP, CNM, LCB, RMM, MJM, DM, RB: critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol conducted in Merino ewes was approved by the Animal Ethics Committee of the Florey Institute of Neuroscience and Mental Health under guidelines laid down by the National Health and Medical Research Council of Australia (20-040-FINMH; 7th September 2020).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of enzyme-linked immunosorbent assay reagents. Figure S1. Silk snare, cannula for infusion and outer tube surgically implanted bilaterally on splanchnic sympathetic nerves.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peiris, R.M., May, C.N., Booth, L.C. et al. Splanchnic sympathetic nerve denervation improves bacterial clearance and clinical recovery in established ovine Gram-negative bacteremia. ICMx 11, 53 (2023). https://doi.org/10.1186/s40635-023-00530-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00530-6