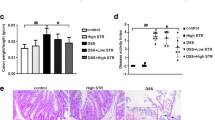
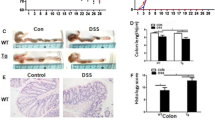
Abstract
Background and Aims
Myosin light chain kinase (MLCK) plays a central role in the mechanisms of barrier dysfunction, and intestinal epithelial MLCK protein expression is upregulated in active ulcerative colitis (UC). ML-7, a MLCK inhibitor, has been used in many MLCK studies. However, the effect of ML-7 has never been estimated in colitis models. The aim of this study was to determine whether ML-7 can treat UC.
Methods
Experimental colitis was induced and ML-7 was administered by intraperitoneal injection. The disease activity index (DAI) scores were evaluated and colon tissue was collected for the assessment of histological changes, myeloperoxidase (MPO) activity, and tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-13 and interleukin (IL)-17 levels. The small intestinal mucosa was ultrastructurally examined, epithelial MLCK protein expression and enzymatic activity were determined, and intestinal permeability was assayed using FITC-dextran 4000 (FD-4) and Evans blue (EB).
Results
ML-7 was found to be significantly effective in reducing the DAI scores and histological index scores, and decreasing MPO activity and TNF-α, IFN-γ, IL-13 and IL-17 levels. The small intestinal epithelial MLCK protein expression and enzymatic activity were downregulated by ML-7. The epithelial cells and intercellular tight junctions were ameliorated, and the amount of FD-4 in blood and EB permeating into the intestine were decreased by ML-7 in colitis mice.
Conclusions
ML-7 has a significant anti-colitis effect in colitis mice. It is mainly associated with the inhibition of the epithelial MLCK protein expression, resulting in ameliorated intestinal mucosal permeability.





Similar content being viewed by others
References
Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163–1169.
D’Incà R, Annese V, di Leo V, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1455–1461.
Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347.
Hilsden RJ, Meddings JB, Hardin J, et al. Intestinal permeability and postheparin plasma diamine oxidase activity in the prediction of Crohn’s disease relapse. Inflamm Bowel Dis. 1999;5:85–91.
Schwarz BT, Wang F, Shen L, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394.
Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, et al. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na(+)-glucose cotransport. Am J Physiol. 2001;281:1487–1493.
Michel A, John C, et al. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2007;292:590–598.
Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804.
Moriez R, Salvador-Cartier C, Theodorou V, et al. Myosin light chain kinase is involved in lipopolysaccharide- induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071–1079.
Scott KG, Meddings JB, Kirk DR, et al. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–1190.
Feighery LM, Cochrane SW, Quinn T, et al. Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro. Pharm Res. 2008;25:1377–1386.
Blair SA, Kane SV, Clayburgh DR, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201.
Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:496–504.
Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331–1346.
Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046.
Zolotarevsky Y, Hecht G, Koutsouris A, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172.
Cury DH, Costa JE, Irika K, et al. Protective effect of octreotide and infliximab in an experimental model of indomethacin-induced inflammatory bowel disease. Dig Dis Sci. 2008;53:2516–2520.
Kihara N, de la Fuente SG, Fujino K, et al. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719.
Kannengiesser K, Maaser C, Heidemann J, et al. Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:324–331.
Bansal V, Costantini T, Kroll L, et al. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. 2009;26:1353–1359.
Berruet N, Sentenac S, Auchere D, et al. Effect of efavirenz on intestinal p-glycoprotein and hepatic p450 function in rats. J Pharm Pharm Sci. 2005;8:226–234.
van Sommeren S, Visschedijk MC, Festen EA, et al. HNF4α and CDH1 are associated with ulcerative colitis in a Dutch cohort. Inflamm Bowel Dis. 2011;17:1714–1718.
McGuckin MA, Eri R, Simms LA, et al. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113.
Fries W, Muja C, Crisafulli C, et al. Infliximab and etanercept are equally effective in reducing enterocyte APOPTOSIS in experimental colitis. Int J Med Sci. 2008;5:169–180.
Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383.
Okayasu I, Hatakeyama S, Yamada M, et al. A novel method of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Gaudio E, Taddei G, Vetuschi A, et al. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475.
Elson CO, Sartor RB, Tennyson GS, et al. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367.
Mennigen R, Nolte K, Rijcken E, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:1140–1149.
Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Res. 2009;92:2400–2408.
Matsuda R, Koide T, Tokoro C, et al. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naïve ulcerative colitis during active and quiescent disease. Inflamm Bowel Dis. 2009;15:328–334.
Olsen T, Goll R, Cui G, et al. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42:1312–1320.
Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564.
Ajduković J, Tonkić A, Salamunić I, et al. Interleukins IL-33 and IL-17/IL-17A in patients with ulcerative colitis. Hepatogastroenterology. 2010;57:1442–1444.
Kamada N, Hisamatsu T, Honda H, et al. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn’s disease. Inflamm Bowel Dis. 2010;16:568–575.
Førland DT, Johnson E, Saetre L, et al. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on expression of cytokines and calprotectin in patients with ulcerative colitis and Crohn’s disease. Scand J Immunol. 2011;73:66–75.
Wang F, Graham WV, Wang Y, et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419.
Matsumura K, Nakase H, Yamamoto S, et al. Modulation of the Th1/Th2 balance by infliximab improves hyperthyroidism associated with a flare-up of ulcerative colitis. Inflamm Bowel Dis. 2009;15:967–968.
Kurtovic J, Segal I. Recent advances in biological therapy for inflammatory bowel disease. Trop Gastroenterol. 2004;25:9–14.
Nishimoto N, Nakahara H, Yoshio-Hoshino N, et al. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197–1200.
Reenaers C, Louis E, Belaiche J. Current directions of biologic therapies in inflammatory bowel disease. Therap Adv Gastroenterol.. 2010;3:99–106.
Sandborn WJ. Is there a role for infliximab in the treatment of severe ulcerative colitis?: The American experience. Inflamm Bowel Dis. 2008;2:232–233.
Shields CJ, Winter DC. Infliximab for ulcerative colitis. N Engl J Med. 2006;354:1424–1426.
Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:1308.
Ma TY, Anderson JM. Tight junctions and the intestinal barrier. In: Johnson LR, ed. Physiology of the gastrointestinal tract. 4th ed. Philadelphia, PA: Elsevier Science & Technology; 2006.
Ma TY, Boivin MA, Ye D, et al. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:422–430.
Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126.
Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563.
Acknowledgments
Supported by a grant from the First Affiliated Hospital of Anhui Medical University. We thank Professor Ke Chen, Professor Wen Hu and Professor Daobing Wang for assistance with pathological technology, and we also thank Yuxian Shen, MD, PhD, Yongmei Hu, MD, Jiajia Wang, MD, and Qing Zhou, MD, for their support during the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Xu, J., Mei, Q. et al. Myosin Light Chain Kinase Inhibitor Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice. Dig Dis Sci 58, 107–114 (2013). https://doi.org/10.1007/s10620-012-2304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2304-3