Abstract
Background
The induction of prolonged choline-deprivation (CD) in rats receiving thioacetamide (TAA) is an experimental approach of mild hepatotoxicity that could resemble commonly presented cases in clinical practice (in which states of malnutrition and/or alcoholism are complicated by the development of other liver-associated diseases).
Aim
The present study aimed to investigate the time-dependent effects of a 30-, a 60- and a 90-day dietary CD and/or TAA administration on the adult rat liver histopathology and the serum markers of hepatic functional integrity.
Methods
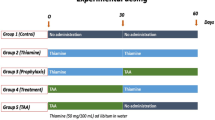
Rats were divided into four main groups: (a) control, (b) CD, (c) TAA and (d) CD + TAA. Dietary CD was provoked through the administration of choline-deficient diet, while TAA administration was performed ad libitum through the drinking water (300 mg/l of drinking water).
Results
Histological examination of the CD + TAA liver sections revealed micro- and macro-vesicular steatosis with degeneration and primary fibrosis at day 30, to extensive steatosis and fibrosis at day 90. Steatosis was mostly of the macrovesicular type, involving all zones of the lobule, while inflammatory infiltrate consisted of foci of acute and chronic inflammatory cells randomly distributed in the lobule. These changes were accompanied by gradually increasing mitotic activity, as well as by a constantly high alpha-smooth muscle actin immunohistochemical staining. The determination of hepatocellular injury markers such as the serum enzyme levels’ of alanine aminotransferase and aspartate aminotransferase demonstrated a decrease at day 30 (they returned to control levels at days 60 and 90). However, the determination of those serum enzymes used for the assessment of cholestatic liver injury (gamma-glutamyltransferase, alkaline phosphatase) revealed a constant (time-independent) statistically-significant increase versus control values.
Conclusions
Long-term combined dietary CD and TAA administration could be a more realistic experimental approach to human liver diseases involving severe steatosis, fibrosis, stellate cell activation and significant regenerative hepatocellular response.



Similar content being viewed by others
Notes
Ingredients of the CDD: sucrose, coconut oil, starch wheat, dextrine, extracted peanut meal, soy protein, corn oil, dicalcium phosphate, cellulose, potassium citrate, sodium chloride, magnesium oxide, l-cystine, vitamin A, vitamin D3, vitamin E (alpha-tocopherol), copper (copper sulfate pentahydrate), selenium (sodium selenite). Analysis: protein (12 %), fat (16 %), fiber (2 %), ash (3.5 %).
References
Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795.
Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296.
Buchman AL, Dubin M, Jenden D, et al. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology. 1992;102:1363–1370.
Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev. 1994;52:327–339.
Liapi C, Feskou I, Zarros A, Galanopoulou P, Tsakiris S. Effects of gestational and lactational choline deprivation on brain antioxidant status, acetylcholinesterase (Na+, K+)- and Mg2+-ATPase activities in offspring rats. Clin Chem Lab Med. 2007;45:651–656.
Zeisel SH. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr. 1981;1:95–121.
Zeisel SH. Choline: an essential nutrient for humans. Nutrition. 2000;16:669–671.
da Costa KA, Cochary EF, Blusztajn JK, Garner SC, Zeisel SH. Accumulation of 1,2-sn-diradylglycerol with increased membrane associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J Biol Chem. 1993;268:2100–2105.
Lombardi B, Pani P, Schlunk FF. Choline-deficiency fatty liver: impaired release of hepatic triglycerides. J Lipid Res. 1968;9:437–446.
Buchman AL, Dubin MD, Moukarzel AA, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403.
Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood). 2004;229:988–995.
Konstandi M, Segos D, Galanopoulou P, et al. Effects of choline-deprivation on paracetamol- or phenobarbital-induced rat liver metabolic response. J Appl Toxicol. 2009;29:101–109.
Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526–1533.
Fernández I, Fontana L, Gil A, Ríosc A, Torres MI. Dietary supplementation with monounsaturated and long-chain polyunsaturated fatty acids influences the liver structural recovery and hepatocyte binuclearity in female Wistar rats in experimental cirrhosis induced by thioacetamide. Exp Toxicol Pathol. 2005;57:65–75.
Pérez MJ, Sánchez-Medina F, Torres M, Gil A, Suárez A. Dietary nucleotides enhance the liver redox state and protein synthesis in cirrhotic rats. J Nutr. 2004;134:2504–2508.
Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–636.
Zimmermann T, Franke H, Dargel R. Biochemical and substructural studies on hepatic and serum lipoprotein metabolism after acute liver injury induced by thioacetamide in rats. Exp Pathol. 1985;28:225–233.
Pérez MJ, Suárez A, Gómez-Capilla JA, Sánchez-Medina F, Gil A. Dietary nucleotide supplementation reduces thioacetamide-induced liver fibrosis in rats. J Nutr. 2002;132:652–657.
Wasser S, Tan CE. Experimental models of hepatic fibrosis in the rat. Ann Acad Med Singap. 1999;28:109–111.
Fernández I, Torres I, Moreira E, Fontana L, Gil A, Rios A. Influence of administration of long-chain polyunsaturated fatty acids on process of histological recovery in liver cirrhosis produced by oral intake of thioacetamide. Dig Dis Sci. 1996;41:197–207.
Moreira E, Fontana L, Periago JL, Sanchéz De Medina F, Gil A. Changes in fatty acid composition of plasma, liver microsomes, and erythrocytes in liver cirrhosis induced by oral intake of thioacetamide in rats. Hepatology. 1995;21:199–206.
Torres-López MI, Fernandez I, Fontana L, Gil A, Rios A. Influence of dietary nucleotides on liver structural recovery and hepatocyte binuclearity in cirrhosis induced by thioacetamide. Gut. 1996;38:260–264.
Zimmermann T, Müller A, Machnik G, Franke H, Schubert H, Dargel R. Biochemical and morphological studies on production and regression of experimental liver cirrhosis induced by thioacetamide in Uje: WIST rats. Z Versuchstierkd. 1987;30:165–180.
Al-Bader A, Mathew TC, Abul H, Al-Sayer H, Singal PK, Dashti HM. Cholangiocarcinoma and liver cirrhosis in relation to changes due to thioacetamide. Mol Cell Biochem. 2000;208:1–10.
Avni Y, Shirin H, Aeed H, Shahmurov M, Birkenfeld S, Bruck R. Thioacetamide-induced hepatic damage in a rat nutritional model of steatohepatitis. Hepatol Res. 2004;30:141–147.
Zarros A, Theocharis S, Skandali N, Tsakiris S. Effects of fulminant hepatic encephalopathy on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na+, K+)- and Mg2+-ATPase: comparison of the enzymes’ response to in vitro treatment with ammonia. Metab Brain Dis. 2008;23:255–264.
Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883–889.
Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int. 2011;31:461–473.
Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–198.
Al-Humadi H, Zarros A, Kyriakaki A, Al-Saigh R, Liapi C. Choline deprivation: an overview of the major hepatic metabolic response pathways. Scand J Gastroenterol. 2012. doi:10.3109/00365521.2012.685755.
Jordao AA, Zanutto ME, Domenici FA, et al. Progression of lipid peroxidation measured as thiobarbituric acid reactive substances, damage to DNA and histopathological changes in the liver of rats subjected to a methionine–choline-deficient diet. Basic Clin Pharmacol Toxicol. 2009;105:150–155.
King J. The transferase-alanine and aspartate transaminase. In: Van D, ed. Practical Clinical Enzymology. London: Nostrand; 1965:121–138.
Persijn JP, van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem. 1976;14:421–427.
Walter K, Schutt C. Acid and alkaline phosphatase in serum (two point method). In: Bergmeyer HU, ed. Methods in Enzymatic Analysis, vol. 2. London: Academic Press; 1974:856–860.
Liapi C, Feskou I, Zarros A, Carageorgiou H, Galanopoulou P, Tsakiris S. Equilibrated diet restores the effects of early age choline-deficient feeding on rat brain antioxidant status and enzyme activities: the role of homocysteine, l-phenylalanine and l-alanine. Metab Brain Dis. 2008;23:289–301.
Best CH, Huntsman ME. The effects of the components of lecithine upon deposition of fat in the liver. J Physiol. 1932;75:405–412.
Hironaka K, Sakaida I, Uchida K, Okita K. Correlation between stellate cell activation and serum fibrosis markers in choline-deficient l-amino acid-defined diet-induced rat liver fibrosis. Dig Dis Sci. 2000;45:1935–1943.
Nieto N, Rojkind M. Repeated whiskey binges promote liver injury in rats fed a choline-deficient diet. J Hepatol. 2007;46:330–339.
Chandar N, Amenta J, Kandala JC, Lombardi B. Liver cell turnover in rats fed a choline-devoid diet. Carcinogenesis. 1987;8:669–673.
Veteläinen R, Bennink RJ, van Vliet AK, van Gulik TM. Mild steatosis impairs functional recovery after liver resection in an experimental model. Br J Surg. 2007;94:1002–1008.
Abanobi SE, Lombardi B, Shinozuka H. Stimulation of DNA synthesis and cell proliferation in the liver of rats fed a choline-devoid diet and their suppression by phenobarbital. Cancer Res. 1982;42:412–415.
Albright CD, Zeisel SH. Choline deficiency causes increased localization of transforming growth factor-beta1 signaling proteins and apoptosis in the rat liver. Pathobiology. 1997;65:264–270.
Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem. 2005;15:59–68.
Albright CD, Liu R, Bethea TC, Da Costa KA, Salganik RI, Zeisel SH. Choline deficiency induces apoptosis in SV40-immortalized CWSV-1 rat hepatocytes in culture. FASEB J. 1996;10:510–516.
da Costa KA, Niculescu MD, Craciunescu CN, Fischer LM, Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94.
Müller D, Sommer M, Kretzschmar M, et al. Lipid peroxidation in thioacetamide-induced macronodular rat liver cirrhosis. Arch Toxicol. 1991;65:199–203.
Abul H, Mathew TC, Dashti HM, Al-Bader A. Level of superoxide dismutase, glutathione peroxidase and uric acid in thioacetamide-induced cirrhotic rats. Anat Histol Embryol. 2002;31:66–71.
Cruz A, Padillo FJ, Torres E, et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J Pineal Res. 2005;39:143–150.
Muriel P, Moreno MG. Effects of silymarin and vitamins E and C on liver damage induced by prolonged biliary obstruction in the rat. Basic Clin Pharmacol Toxicol. 2004;94:99–104.
Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Efficacy of urine bile acid as a non-invasive indicator of liver damage in rats. J Toxicol Sci. 2009;34:27–38.
Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–208.
McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255–262.
James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501.
Acknowledgments
This work was supported by the State Scholarships Foundation of the Hellenic Republic (in terms of a scholarship to Dr. Hussam Al-Humadi), as well as by the National and Kapodistrian University of Athens. The authors wish to acknowledge their appreciation to the medical students John Botis, Konstantinos Kalafatakis and Nikolina Skandali for their technical assistance.
Conflict of interest
No conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Humadi, H., Theocharis, S., Dontas, I. et al. Hepatic Injury Due to Combined Choline-Deprivation and Thioacetamide Administration: An Experimental Approach to Liver Diseases. Dig Dis Sci 57, 3168–3177 (2012). https://doi.org/10.1007/s10620-012-2299-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2299-9