Abstract
Background
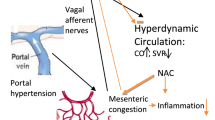
Hepatic encephalopathy is a syndrome whose physiopathology is poorly understood; therefore, current diagnostic tests are imperfect and modern therapy is nonspecific. Particularly, it has been suggested that inflammation plays an important role in the pathogenesis of portal hypertensive encephalopathy in the rat.
Aim
We have studied an experimental model of portal hypertension based on a triple partial portal vein ligation in the rat to verify this hypothesis.
Methods
One month after portal hypertension we assayed in the splanchnic area (liver, small bowel and mesenteric lymph nodes) and in the central nervous system (hippocampus and cerebellum) fractalkine (CX3CL1) and stromal cell-derived factor alpha (SDF1-α) as well as their respective receptors (CX3CR1 and CXCR4) because of their key role in inflammatory processes.
Results
The significant increase of fractalkine in mesenteric lymph nodes (P < 0.05) and its receptor (CX3CR1) in the small bowel (P < 0.05) and hippocampus (P < 0.01), associated with the increased expression of SDF1-α in the hippocampus (P < 0.01) and the cerebellum (P < 0.01) suggest that prehepatic portal hypertension in the rat induces important alterations in the expression of chemokines in the gut-brain axis.
Conclusion
The present study revealed that portal hypertension is associated with splanchnic-brain inflammatory alterations mediated by chemokines.



Similar content being viewed by others
References
Zafirova Z, O’Connor M. Hepatic encephalopathy: current management strategies and treatment, including management and monitoring of cerebral edema and intracraneal hypertension in fulminant hepatic failure. Curr Opin Anaesthesiol. 2010;23:121–127.
Ferenci P, Lockwood A, Muller K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-definition, nomenclature, diagnosis and quantification. Final report of the working party at the 11th World Congress of Gastroenterology, Vienna 1998. Hepatology. 2002;35:716–721.
Sundaram V, Shaikh OS. Hepatic encephalopathy: Physiopathology and emerging therapies. Med Clin N Am. 2009;93:819–836.
Mínguez B, García-Pagán JC, Bosch J, et al. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology. 2006;46:707–714.
Yadav SK, Srivastava A, Srivastava A, et al. Encephalopathy assessment in children with extra-hepatic portal vein obstruction with MR, psychometry and critical flicker frequency. J Hepatol. 2010;52:348–354.
Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Pharmacol. 1985;248:G618–G625.
Abraldes JG, Pasarin M, García-Pagán JC. Animal models of portal hypertesion. World J Gastroenterol. 2006;12:6577–6584.
Aller MA, Méndez M, Nava MP, López L, Arias JL, Arias J. The value of microsurgery in liver research. Liver Int. 2009;29:1132–1140.
Aller MA, Méndez M, Nava MP, et al. Portal surgery: Portosystemic shunts and portal hypertension. In: Aller MA, Arias J, eds. Microsurgery in Liver Research. Bentham Scientific e-books. 2009;117–136.
Shawcross D, Jalan R. The physiopathological basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–2304.
Shawcross DL, Wright G, Olde Damink SWM, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125–138.
Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062–1069.
Arias JL, Aller MA, Sánchez-Patán F, Arias J. The inflammatory bases of hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2006;18:1297–1310.
Aller MA, Arias JL, Cruz A, Arias J. Inflammation: a way to understanding the evolution of portal hypertension. Theor Biol Med Model. 2007;4:44–69.
Bendall L. Chemokines and their receptors in disease. Histol Histopathol. 2005;20:907–926.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621.
Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55.
Monterde G, Rodríguez-Fabián G, Vara E, et al. Increased plasma levels of corticosterone and prolactine and decreased T3 and T4 levels in short-term prehepatic portal hypertension in rats. Dig Dis Sci. 2000;45:1865–1871.
Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Compact 2nd Edition. London: Academic Press; 1997.
Lynch MA, Voss KL. Presynaptic changes in long-term potentiation: elevated synaptosomal calcium concentration and basal phosphoinositide turnover in dentate gyrus. J Neurochem. 1991;1:113–118.
Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254.
Merino JJ, Cordero MI, Sandi C. Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. Eur J Neurosci. 2000;12:3283–3290.
Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339.
Coelho AL, Hogaboam CM, Kunkel SL. Chemokines provide the sustained inflammatory bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005;16:553–560.
Aller MA, Arias JL, Arias J. The mast cell integrates the splanchnic and systemic inflammatory response in portal hypertension. J Transl Med. 2007;5:44–59.
Díez-Arias JA, Aller MA, Palma MD, et al. Increased duodenal mucosa infiltration by mast cells in rats with portal hypertension. Dig Surg. 2001;18:34–40.
Llamas MA, Aller MA, Marquina D, Nava MP, Arias J. Bacterial translocation to mesenteric lymph nodes increases in chronic portal hypertensive rats. Dig Dis Sci. 2010;55:2244–2254.
Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2006;217:304–328.
Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258.
Alonso MJ, Aller MA, Corcuera MT, et al. Progressive hepatocytic fatty infiltration in rats with prehepatic portal hypertension. Hepatogastroenterology. 2005;52:541–546.
Simpson KJ, Henderson NC, Bone-Larson CL, Lukacs NW, Hogaboam CM, Kunkel SL. Chemokines in the pathogenesis of liver disease: so many players with poorly defined roles. Clin Sci. 2003;104:47–63.
Palma MD, Aller MA, Vara E, et al. Portal hypertension produces an evolutive hepato-intestinal pro- and anti-inflammatory response in the rat. Cytokine. 2005;31:213–226.
Sánchez-Patán F, Anchuelo R, Aller MA, et al. Chronic prehepatic portal hypertension in the rat: is it a type of metabolic inflammatory syndrome? Lipids Health Dis. 2008;7:4–13.
Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: Pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894.
Kasiyanov A, Fujii N, Tamamura H, Xiong H. Modulation of network-driven, GABA-mediated giant depolarizing potentials by SDF-1alpha in the developing hippocampus. Dev Neurosci. 2008;30:285–292.
Barbieri F, Bajetto A, Porcile C, Pattarozzi A, Schettini G, Florio T. Role of stromal cell-derived factor 1 (SDF1/CXCL12) in regulating anterior pituitary function. J Mol Endocrinol. 2007;38:383–389.
Guerin E, Sheridan C, Assheton D, et al. SDF-1-alpha is associated with VEGFR-2 in human choroidal neovascularization. Microvasc Res. 2008;75:302–307.
Guyon A, Nahon J-L. Multiple actions of the chemokine stromal cell-derived factor-1α on neuronal activity. J Mol Endocrinol. 2007;38:365–376.
Bertollini C, Ragozzino D, Gross C, Limatola C, Eusebi F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology. 2006;51:816–821.
Butterworth RF. Pathogenesis of hepatic encephalopathy: new insights from neuroimaging and molecular studies. J Hepatol. 2003;29:278–285.
Weissenborn K, Bokemeyer M, Ahl B, et al. Functional imaging of the brain in patients with liver cirrhosis. Metab Brain Dis. 2004;19:269–280.
Weissenborn K, Giewekemeyer K, Heidenreich S, Bokemeyer M, Berding G, Ahl B. Attention, memory and cognitive function in hepatic encephalopathy. Metab Brain Dis. 2005;20:359–367.
Lores-Arnaiz S, Perazzo JC, Prestifilipo JP, et al. Hippocampal mitochondrial dysfunction with decreased mtNOS activity in prehepatic portal hypertensive rats. Neurochem Int. 2005;47:362–368.
Acosta GB, Fernández MA, Rosello DM, Tomaro ML, Balestrasse K, Lemberg A. Glutamine synthetase activity and glutamate uptake in hippocampus and frontal cortex in portal hypertensive rats. World J Gastroenterol. 2009;15:2893–2899.
Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anat Rec. 2009;292:1914–1921.
Méndez M, Méndez-López M, López L, et al. Spatial memory alterations in three models of hepatic encephalopathy. Behav Brain Res. 2008;188:32–40.
Méndez M, Méndez-López M, López L, Aller MA, Arias J, Arias JL. Working memory impairment and reduced hippocampal and prefrontal cortex c-Fos expression in a rat model of cirrhosis. Physiol Behav. 2008;95:302–307.
Santin LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–150.
Méndez M, Méndez-López M, López L, Aller MA, Arias J, Arias JL. Mammillary body alterations and spatial memory impairment in Wistar rats with thioacetamide-induced cirrhosis. Brain Res. 2008;1233:185–195.
Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122–1132.
Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354.
Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765.
De Haas AH, Van Weering HRJ, De Jong EK, Boddeke HWGM, Biber KPH. Neuronal chemokines: Versatile messengers in central nervous system cell interaction. Mol Neurobiol. 2007;36:137–151.
Lauro C, Di Angelantonio S, Cipriani R, et al. Activity of adenosin receptors Type 1 is required for CX3CL-1-mediated neuroprotection and neuromodulation. J Immunol. 2008;180:7590–7596.
Wikgren J, Nokia MS, Penttonen M. Hippocampo-cerebellar theta band phase synchrony in rabbits. Neuroscience. 2010;165:1538–1545.
Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224.
Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. PNAS. 2002;99:7090–7095.
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599.
Martino G. How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS-disorders. Lancet Neurol. 2004;3:372–378.
Martell M, Coll M, Ezkurdia N, Raurell I, Genesca J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208–220.
Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. PNAS. 2000;97:8075–8080.
Juremalm M, Nilsson G. Chemokine receptor expression by mast cells. Chem Immunol Allergy. 2005;87:130–144.
Bischoff SC. Physiological and physiopathological functions of intestinal mast cells. Semin Immunopathol. 2009;31:185–205.
Acknowledgments
We are indebted to Maria Elena Vicente for her invaluable assistance in preparing the manuscript and Elizabeth Mascola for translating it. This study was supported in part with grants from the “Mutua Madrileña” Research Foundation (FMM Ref. No. AP69772009 and Ref. No. AP59662009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merino, J., Aller, MA., Rubio, S. et al. Gut-Brain Chemokine Changes in Portal Hypertensive Rats. Dig Dis Sci 56, 2309–2317 (2011). https://doi.org/10.1007/s10620-011-1625-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1625-y