Abstract
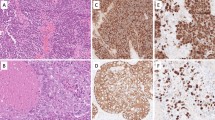
Pleiotrophin (PTN), a heparin-binding growth factor also known as neurite growth-promoting factor, exhibits several properties related with tumor development. PTN and its receptor, N-syndecan, may play a very important role in tumor growth and neural invasion of pancreatic cancer. We investigated PTN and N-syndecan protein levels in 38 patients with pancreatic cancer by immunohistochemistry, and analyzed for its correlation with clinicopathological features, perineural invasion, and prognosis. The results showed that PTN and N-syndecan proteins were found in 24 (63.2%) and 22 (57.9%) specimens, respectively. PTN and N-syndecan expressions were associated with perineural invasion (P = 0.016 and P = 0.029, respectively). High PTN expression was closely related to an advanced TNM stage (P = 0.007), lymph node metastasis (P = 0.040), and decreased postoperative survival at 3 years (50.0% versus 20.8%, respectively; P = 0.001). We conclude that high expression of PTN combined with N-syndecan may contribute to the increased perineural invasion and poor prognosis of pancreatic cancer.


Similar content being viewed by others
References
Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M (2003) Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 27:324–329. doi:10.1007/s00268-002-6659-z
Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW (2004) Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 91:586–594. doi:10.1002/bjs.4484
Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363:1049–1057. doi:10.1016/S0140-6736(04)15841-8
Laheru D, Jaffee EM (2005) Immunotherapy for pancreatic cancer—science driving clinical progress. Nat Rev Cancer 5:459–467. doi:10.1038/nrc1630
Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H et al (2002) Perineural invasion in pancreatic cancer. Pancreas 24:15–25. doi:10.1097/00006676-200201000-00003
Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T et al (2006) The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg 244:274–281. doi:10.1097/01.sla.0000217642.68697.55
Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P et al (2004) Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res 64:5291–5300. doi:10.1158/0008-5472.CAN-04-1112
Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T et al (2003) Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur J Neurosci 17:2127–2134. doi:10.1046/j.1460-9568.2003.02661.x
Bao X, Mikami T, Yamada S, Faissner A, Muramatsu T, Sugahara K (2005) Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J Biol Chem 280:9180–9191. doi:10.1074/jbc.M413423200
Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY (2002) Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys 397:162–171. doi:10.1006/abbi.2001.2705
Laaroubi K, Delbe J, Vacherot F, Desgranges P, Tardieu M, Jaye M et al (1994) Mitogenic and in vitro angiogenic activity of human recombinant heparin affin regulatory peptide. Growth Factors 10:89–98. doi:10.3109/08977199409010982
Muramatsu T (2002) Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132:359–371
Peria FM, Neder L, Marie SK, Rosemberg S, Oba-Shinjo SM, Colli BO et al (2007) Pleiotrophin expression in astrocytic and oligodendroglial tumors and its correlation with histological diagnosis, microvascular density, cellular proliferation and overall survival. J Neurooncol 84:255–261. doi:10.1007/s11060-007-9379-2
Kadomatsu K, Muramatsu T (2004) Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204:127–143. doi:10.1016/S0304-3835(03)00450-6
Lu KV, Jong KA, Kim GY, Singh J, Dia EQ, Yoshimoto K et al (2005) Differential induction of glioblastoma migration and growth by two forms of pleiotrophin. J Biol Chem 280:26953–26964. doi:10.1074/jbc.M502614200
Muller S, Kunkel P, Lamszus K, Ulbricht U, Lorente GA, Nelson AM et al (2003) A role for receptor tyrosine phosphatase zeta in glioma cell migration. Oncogene 22:6661–6668. doi:10.1038/sj.onc.1206763
Wu H, Barusevicius A, Babb J, Klein-Szanto A, Godwin A, Elenitsas R et al (2005) Pleiotrophin expression correlates with melanocytic tumor progression and metastatic potential. J Cutan Pathol 32:125–130. doi:10.1111/j.0303-6987.2005.00282.x
Chen H, Gordon MS, Campbell RA, Li M, Wang CS, Lee HJ et al (2007) Pleiotrophin is highly expressed by myeloma cells and promotes myeloma tumor growth. Blood 110:287–295. doi:10.1182/blood-2006-08-042374
Chang Y, Zuka M, Perez-Pinera P, Astudillo A, Mortimer J, Berenson JR et al (2007) Secretion of pleiotrophin stimulates breast cancer progression through remodeling of the tumor microenvironment. Proc Natl Acad Sci USA 104:10888–10893. doi:10.1073/pnas.0704366104
Weber D, Klomp HJ, Czubayko F, Wellstein A, Juhl H (2000) Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res 60:5284–5288
Klomp HJ, Zernial O, Flachmann S, Wellstein A, Juhl H (2002) Significance of the expression of the growth factor pleiotrophin in pancreatic cancer patients. Clin Cancer Res 8:823–827
Souttou B, Juhl H, Hackenbruck J, Rockseisen M, Klomp HJ, Raulais D et al (1998) Relationship between serum concentrations of the growth factor pleiotrophin and pleiotrophin-positive tumors. J Natl Cancer Inst 90:1468–1473. doi:10.1093/jnci/90.19.1468
Kinnunen A, Kinnunen T, Kaksonen M, Nolo R, Panula P, Rauvala H (1998) N-syndecan and HB–GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur J Neurosci 10:635–648. doi:10.1046/j.1460-9568.1998.00082.x
Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H (1994) Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB–GAM). Identification as N-syndecan (syndecan-3). J Biol Chem 269:12999–13004
Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H (1998) Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem 273:10702–10708. doi:10.1074/jbc.273.17.10702
Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M et al (1999) Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol 17:2419–2428
Wittekind C, Compton CC, Greene FL, Sobin LH (2002) TNM residual tumor classification revisited. Cancer 94:2511–2516. doi:10.1002/cncr.10492
Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T et al (1999) The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today 29:16–22. doi:10.1007/BF02482964
Nakao A, Harada A, Nonami T, Kaneko T, Takagi H (1996) Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas 12:357–361. doi:10.1097/00006676-199605000-00006
Kayahara M, Nakagawara H, Kitagawa H, Ohta T (2007) The nature of neural invasion by pancreatic cancer. Pancreas 35:218–223
Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H (1997) Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol 65:164–170. doi:10.1002/(SICI)1096-9098(199707)65:3<164::AID-JSO4>3.0.CO;2-4
Miyazaki I (1997) Perineural invasion and surgical treatment of the pancreas head cancer. Nippon Geka Gakkai Zasshi 98:646–648
Sudo T, Murakami Y, Uemura K, Hayashidani Y, Hashimoto Y, Ohge H, et al (2008) Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci
Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N et al (2007) Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol 31:1636–1644
Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A et al (2003) Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas 27:225–229. doi:10.1097/00006676-200310000-00005
Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S et al (2000) Heparin-binding proteins HB–GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol 19:377–387. doi:10.1016/S0945-053X(00)00084-6
Acknowledgments
Supported by the Program of Science & Technology of Shanxi Province (No. 2007K09-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, J., Ma, Q., Wang, L. et al. Pleiotrophin Expression in Human Pancreatic Cancer and Its Correlation with Clinicopathological Features, Perineural Invasion, and Prognosis. Dig Dis Sci 54, 895–901 (2009). https://doi.org/10.1007/s10620-008-0433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0433-5