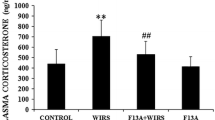
Abstract
Sensory neuron activation reduces water-immersion restraint stress (WIR)-induced gastric mucosal injury by inhibiting neutrophil activation through increase in endothelial production of prostacyclin. This study was designed to examine whether lafutidine, which is an H2-receptor antagonist and activates sensory neurons, inhibits neutrophil activation, thereby reducing WIR-induced gastric mucosal injury. Lafutidine enhanced WIR-induced increases in gastric tissue levels of calcitonin gene-related peptide (CGRP) and 6-keto-PGF1α, a stable metabolite of prostacyclin, whereas famotidine, another H2-receptor antagonist, did not. Such lafutidine-induced increases in gastric tissue levels of 6-keto-PGF1α were reversed by pretreatment with capsazepine, an inhibitor of sensory neuron activation, CGRP(8–37), a CGRP antagonist, and indomethacin. Lafutidine inhibited acid-induced exacerbation of gastric mucosal injury in animals subjected to WIR by inhibiting neutrophil activation, whereas famotidine did not. Lafutidine synergistically increased CGRP release from isolated rat dorsal root ganglion neurons in the presence of anandamide, but famotidine did not. These observations suggest that lafutidine might reduce WIR-induced gastric mucosal injury not only by inhibiting acid secretion but also by inhibiting neutrophil activation through enhancement of sensory neuron activation.







Similar content being viewed by others
References
Holzer P, Guth PH (1991) Neuropeptide control of rat gastric mucosal blood flow: increase by calcitonin gene-related peptide and vasoactive intestinal polypeptide, but not substance P and neurokinin A. Circ Res 12:25–31
Holzer P (2000) Local microcirculatory reflexes and afferent signalling in response to gastric acid challenge. Gut 47:46–48
Sobue M, Joh T, Oshima T, Suzuki H, Seno K, Kasugai K, Nomura T, Ohara H, Yokoyama Y, Itoh M (2003) Contribution of capsaicin-sensitive afferent nerves to rapid recovery from ethanol-induced gastric epithelial damage in rats. J Gastroenterol Hepatol 18:1188–1195
Robert A (1979) Cytoprotection by prostaglandins. Gastroenterology 77:761–767
Konturek SJ, Robert A (1982) Cytoprotection of canine gastric mucosa by prostacyclin: Possible mediation by increased mucosal blood flow. Digestion 25:155–163
Hamajima E, Sugiyama S, Hoshino H, Goto H, Tsukamoto Y, Ozawa T (1994) Effect of FK506, an immunosuppressive agent, on genesis of water-immersion stress-induced gastric lesions in rats. Dig Dis Sci 39:713–720
Wallace JL, Keenan CM, Granger DN (1990) Gastric ulceration induced by nonsteroidal antiinflammatory drugs is a neutrophil-dependent process. Am J Physiol 259:G462–G467
Kushimoto S, Okajima K, Okabe H, Binder BR (1996) Role of granulocyte elastase in the formation of hemorrhagic shock-induced gastric mucosal lesions in the rat. Crit Care Med 24:1041–1046
Weiss SJ, LoBuglio AF (1980) An oxygen-dependent mechanism of neutrophil-mediated cytotoxicity. Blood 55:1020–1024
Weiss SJ, Young J, LoBuglio AF, Slivka A, Nimeh NF (1981) Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest 68:714–721
Szallasi A, Blumberg PM (1996) Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain 68:195–208
Kainoh M, Imai R, Umetsu T, Hattori M, Nishio S (1990) Prostacyclin and beraprost sodium as suppressors of activated rat polymorphonuclear leukocytes. Bichem Pharmacol 39:477–484
Simpson PJ, Mickelson J, Fantone JC, Gallagher KP, Lucchesi BR (1987) Iloprost inhibits neutrophil function in vitro and in vivo and limits experimental infarct size in canine heart. Circ Res 60:666–673
Harada N, Okajima K, Murakami K, Isobe H, Liu W (1999) Gastric prostacyclin (PGI2) prevents stress-induced gastric mucosal injury in rats primarily by inhibiting leukocyte activation. Prostaglandins Other Lipid Mediat 57: 291–303
Harada N, Okajima K, Liu W, Uchiba M (2000) Activated neutrophils impair gastric cytoprotection. Role of neutrophil elastase. Dig Dis Sci 45:1210–1216
Harada N, Okajima K, Uchiba M, Katsuragi T (2003) Contribution of capsaicin-sensitive sensory neurons to stress-induced increases in gastric tissue levels of prostaglandins in rats. Am J Physiol Gastrointest Liver Physiol 285:G1214–G1224
Sato H, Kawashima K, Yuki M, Kazumori H, Azharul M, Rumi K, Ortega-Cava CF, Ishihara S, Kinoshita Y (2003) Lafutidine, a novel histamine H2-receptor antagonist, increases serum calcitonin gene-related peptide in rats after water immersion-restraint stress. J Lab Clin Med 141:102–105
Shimatani T, Inoue M, Kuroiwa T, Xu J, Nakamura M, Tazuma S, Ikawa K, Morikawa N (2006) Lafutidine, a newly developed antiulcer drug, elevates postprandial intragastric pH and increases plasma calcitonin gene-related peptide and somatostatin concentrations in humans: comparisons with famotidine. Dig Dis Sci 51:114–120
Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, Priestley JV, Murayama T (2004) Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol 39:303–312
Mimaki H, Kagawa S, Aoi M, Kato S, Satoshi T, Kohama K, Takeuchi K (2002) Effect of lafutidine, a histamine H2-receptor antagonist, on gastric mucosal blood flow and duodenal HCO3- secretion in rats: relation to capsaicin-sensitive afferent neurons. Dig Dis Sci 47:2696–2703
Hisanaga Y, Goto H, Tachi K, Hayakawa T, Sugiyama S (1996) Implication of nitric oxide synthase activity in the genesis of water immersion stress-induced gastric lesions in rats: the protective effects of FK506. Aliment Pharmacol Ther 10:933–940
Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A (1994) Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumor necrosis factor alpha. Gut 35:909–915
Okajima K, Harada N, Uchiba M (2002) Ranitidine reduces ischemi/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J Phamacol Exp Ther 301:1157–1165
Perkins MN, Campbell EA (1992) Capsazepine reversal of the antinociceptive action of capsaicin in vivo. Br J Pharmacol 107:329–333
Kaneko H, Kaunitz J, Taché Y (1998) Vagal mechanisms underlying gastric protection induced by chemical activation of raphe pallidus in rats. Am J Physiol 275:G1056–G1062
Geppetti P, Tramontana M, Evangelista S, Renzi D, Maggi CA, Fusco BM, Del Bianco E (1991) Differential effect of neuropeptide release of different concentrations of hydrogen ions on afferent and intrinsic neurons of the rat stomach. Gastroenterology 101:1505–1511
Sternini C, Reeve JR, Brecha N (1987) Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology 93:852–862
Redfern JS, Lee E, Feldman M (1987) Effect of indomethacin on gastric mucosal prostaglandins in human. Correlation with mucosal damage. Gastroenterology 92:969–977
Wallace JL (1987) Glucocorticoid-induced gastric mucosal damage. Inhibition of leukotriene but not prostaglandin biosynthesis. Prostaglandins 34:311–323
Cooper LC, Dial EJ, Lichtenberger LM (1990) Effect of milk, prostaglandin and antacid in experimentally-induced duodenitis in the rat. Dig Dis Sci 35:1211–1216
Tepperman BL, Besco JM, Kiernan JA (1991) Relationship between myeloperoxidase activity and the ontogenic response of rat gastric mucosa to ethanol. Can J Physiol Pharmacol 69:1882–1888
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster. Gastroenterology 87:1344–1350
Harada N, Okajima K, Yuksel M, Isobe H (2005) Contribution of capsaicin-sensitive sensory neurons to antithrombin-induced reduction of ischemia/repurfusion-induced liver injury in rats. Thromb Haemost 93:48–56
Ahluwalia J, Yaqoob M, Urban L (2003) Activation of capsaicin-sensitive primary sensory neurones induces anandamide production and release. J Neurochem 84:585–591
Okajima K, Murakami K, Liu W, Uchiba M (2000) Inhibition of neutrophil activation by ranitidine contributes to prevent stress-induced gastric mucosal injury in rats. Crit Care Med 28:2858–2865
Holzer P (1998) Neural emergency system in the stomach. Gastroenterology 114:823–839
Harada N, Okajima K, Liu W (2005) Rebamipide decreases the susceptibility of gastric mucosa to acid-induced injury in rats by inhibiting neutrophil activation. Dig Dis Sci 50:S56–S62
Liu W, Okajima K, Murakami K (1998) Role of neutrophil elastase in stress-induced gastric mucosal injury in rats. J Lab Clin Med 132:432–439
Nagy I, Santha P, Jancso G, Urban L (2004) The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol 500:351–369
Fukushima K, Aoi Y, Kato S, Takeuchi K (2006) Gastro-protective action of lafutidine mediated by capsaicin-sensitive afferent neurons without interaction with TRPV1 and involvement of endogenous prostaglandins. World J Gastroenterol 12:3031–3037
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harada, N., Okajima, K. Inhibition of Neutrophil Activation by Lafutidine, an H2-receptor Antagonist, Through Enhancement of Sensory Neuron Activation Contributes to the Reduction of Stress-Induced Gastric Mucosal Injury in Rats. Dig Dis Sci 52, 469–477 (2007). https://doi.org/10.1007/s10620-006-9620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9620-4