Abstract
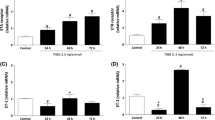
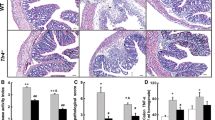
The role of microcirculation in the pathogenesis and course of chronic inflammatory bowel disease is still unclear. The aim of this study was the evaluation of the role of microcirculation in colitis activity in the rat TNBS (trinitrobenzenesulfonic acid) colitis model using endothelin-1 and a selective endothelin-1 receptor antagonist (LU-135252). Target parameters were capillary blood flow, functional capillary density, vascular permeability, and leukocyte sticking as well as recording of hematocrit, weight course, diuresis, stool quality, and degree of inflammation using a histological colitis score. The acute phase of TNBS colitis is characterized by an extensive disturbance of microcirculation (a significant decrease in capillary blood flow and capillary density and a significant increase in capillary permeability and leukocyte sticking in the mucosa). There is also a significant increase in hematocrit and a significant decrease in diuresis and weight. An exogenous supply of endothelin-1 does not lead to an aggravation of these disorders because of a possible blockage of the endothelin-1 receptors by endogenous endothelin-1 in this florid inflammatory phase. Applying the selective endothelin-1 receptor A antagonist LU-135252 leads to a significant improvement of all microcirculatory parameters and clinical findings compared to the untreated colitis group. Direct improvement of capillary blood flow in the early phase of colitis leads to reduced colitis activity, which underscores the pathogenetic role of the microcirculation in the progression of colitis.









Similar content being viewed by others
References
Ginzburg L, Oppenheimer GD (1933) Non-specific granulomata of the intestines. Ann Surg 98:1046–1062
Marston A, Marcuson RW, Chapman M, Arthur JF (1969) Experimental study of devascularization of the colon. Gut 10:121–130
Foitzik T, Kruschewski M, Kroesen A, Buhr HJ (1999) Does microcirculation play a role in the pathogenesis of inflammatory bowel diseases? Int J Colorectal Dis 14:29–34
Kruschewski M, Foitzik T, Perez-Cantó A, Hübotter A, Buhr HJ (2001) Changes of colonic mucosal microcirculation and histology in two colitis models: an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci 46:2336–2343
Laroux FS, Grisham B (2001) Immunological basis of inflammatory bowel disease: role of the microcirculation. Microcirculation 8:283–301
Thornton M, Solomon MJ (2002) Crohn’s disease: in defense of a microvascular aetiology. Int J Colorectal Dis 17:287–297
Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AAM, Pounder RE (1989) Pathogenesis of Crohn’s disease: multifocal gastrointestinal infarction. Lancet 334:1057–1062
Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, Pittilo RM, Rowles PM, Hudson M, Lewis AAM, Pounder RE (1991) Granulomatous vasculitis in Crohn’s disease. Gastroenterology 100:1279–1287
Wakefield AJ, Ekbom A, Dhillon AP, Pittilo RM, Pounder RE (1995) Crohn’s disease: pathogenesis and persistant measles virus infection. Gastroenterology 108:911–916
Fries W, Pagiaro E, Canova E, Carraro P, Gasparini G, Pomerri F, Martin A, Carlotto C, Mazzon E, Sturniolo GC, Longo G (1998) The effect of heparin on trinitrobenzene sulphonic acid-induced colitis in the rat. Aliment Pharmacol Ther 12:229–236
Törkvist L, Thorlacius H, Sjöqvist U, Bohman L, Lapidus A, Flood L, Agren B, Raud J, Löfberg R (1999) Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther 13:1323–1328
Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GSA, Smith OP, Keeling PWN (2000) Randomized comparsion of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther 14:1015–1022
Vrij AA, Jansen JM, Schoon EJ, de Bruine A, Hemker HC, Stockbrügger RW (2001) Low molecular weight heparin treatment in steroid refractory ulcerative colitis: Clinical outcome and influence on mucosal capillary thrombi. Scand J Gastroenterol 36 (Suppl 234):41–47
Brahme F, Lindström C (1970) A comparative radiographic and pathological study of intestinal vasoarchitecture in Crohn’s disease and in ulcerative colitis. Gut 11:928–940
Anthony A, Pounder RE, Dhillon AP, Wakefield AJ (1997) Vascular anatomy defines sites of indomethacin induced jejunal ulceration along the mesenteric margin. Gut 41:763–770
Anthony A, Pounder RE, Dhillon AP, Wakefield AJ (2000) Similarities between ileal Crohn’s disease and indomethacin experimental jejunal ulcers in the rat. Aliment Pharmacol Ther 14:241–245
Menger MD, Lehr H-A (1993) Scope and perspectives of intravital microscopy—bridge over from in vitro to in vivo. Immunol Today 14:519–522
Mithöfer K, Schmidt J, Gebhard MM, Buhr HJ, Herfarth Ch, Klar E (1995) Measurement of blood flow in pancreatic exchange capillaries with FITC-labeled erythrocytes. Microvasc Res 49:33–48
Murch SH, Braegger CP, Sessa WC, MacDonald TT (1992) High endothelin-1 immunoreactivity in Crohn’s disease and ulcerative colitis. Lancet 339:381–385
Hogaboam CM, Muller MJ, Collins SM, Hunt RH (1996) An orally active non-selective endothelin receptor antagonist, bosentan, markedly reduces injury in rat model of colitis. Eur J Pharmacol 309:261–269
Letizia C, Boirivant M, De Toma G, Cerci S, Subioli S, Scuro L, Ferrari P, Pallone F (1998) Plasma levels of endothelin-1 in patients with Crohn’s disease and ulcerative colitis. Ital J Gastroenterol Hepatol 30:266–269
Güllüoğlu BM, Kurtel H, Güllüoğlu MG, Yeğen C, Aktan AÖ, Dizdaroğlu F, Yalin R, Yeğen BÇ (1999) Role of endothelins in trinitrobenzene sulfonic acid-induced colitis in rats. Digestion 60:484–492
Padol I, Huang JQ, Hogaboam CM, Hunt RH (2000) Therapeutic effects of the endothelin receptor antagonist Ro 48-5695 in the TNBS/DNBS rat model of colitis. Eur J Gastroenterol Hepatol 12:257–265
Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K (2001) VEGF, basic–FGF, and TGF–β in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol 96:822–828
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ, Foitzik T (2000) Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut 46:390–394
Foitzik T, Faulhaber J, Hotz HG, Kirchengast M, Buhr HJ (1998) Endothelin receptor blockade improves fluid sequestration, pancreatic capillary blood flow and survival in severe experimental pancreatitis. Ann Surg 228:670–675
Foitzik T, Hotz HG, Eibl G, Hotz B, Kirchengast M, Buhr HJ (1999) Therapy for microcirculatory disorders in severe acute pancreatitis: effectiveness of platelet-activating factor receptor blockade vs. endothelin receptor blockade. J Gastrointest Surg 3:244–251
Foitzik T, Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ (2000) Endothelin receptor blockade in severe acute pancreatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival rates. Surgery 127:399–407
Nolte D, Zeintl H, Steinbauer M, Pickelmann S, Messmer K (1995) Functional capillary density: an indicator of tissue perfusion? Int J Microcirc 15:244–249
Klyscz T, Jünger M, Jung F, Zeintl H (1997) Cap image—-a new kind of computer–assisted video image analysis system for dynamic capillary microscopy. Biomed Technik 42:168–175
Hoffmann JN, Vollmar B, Inthorn D, Schildberg FW, Menger MD (1999) A chronic model for intravital microscopic study of microcirculatory disorders and leukocyte/endothelial cell interaction during normotensive endotoxemia. Shock 12:355–364
Granger DN, Kvietys PR, Perry MA (1993) Leukocyte-endothelial cell adhesion induced by ischemia and reperfusion. Can J Physiol Pharmacol 71:67–75
Granger DN, Kubes P (1994) The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol 55:662–675
Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348:730–732
Traish AM, Moran E, Saenz de Tejada I (1991) Physicochemical characterization and solubilization of endothelin receptors. Receptor 1:229–242
Deniz M, Cetinel S, Kurtel H (2004) Blood flow alterations in TNBS-induced colitis: role of endothelin receptors. Inflamm Res 53:329–336
Shibata Y, Taruishi M, Ashida T (1993) Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid—colonoscopic and histopathologic studies. Gastroenterol Jpn 28:518–527
Wirtz S, Neurath MF (2000) Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis 15:144–160
Malizia G, Calabrese A, Cottone M, Raimondo M, Trejdosiewicz LK, Smart CJ, Oliva L, Pagliaro L (1991) Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology 100:150–159
Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK (1992) Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology 103:840–847
Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H (1993) In situ expression of the cell adhesion molecules in inflammatory bowel disease: evidence of immunologic activation of vascular endothelial cells. Lab Invest 69:77–85
Albelda SM, Smith CW, Ward PA (1994) Adhesion molecules and inflammatory injury. FASEB J 8:504–512
Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, Dixon MF, Axon AT (1995) Adhesion molecules in inflammatory bowel disease. Gut 36:724–730
Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C (1997) Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112:1895–1907
Binion DG, West GA, Volk EE, Drazba JA, Ziats NP, Petras RE, Fiocchi C (1998) Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease. Lancet 352:1742–1746
Panés J, Granger DN (1998) Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 114:1066–1090
Carlos TM, Harlan JM (1994) Leukocyte-endothelial adhesion molecules. Blood 84:2068–2101
Arndt H, Palitzsch K-D, Anderson DC, Rusche J, Grisham MB, Granger DN (1995) Leukocyte-endothelial cell adhesion in a model of intestinal inflammation. Gut 37:374–379
del Zoppo GJ (1997) Microvascular responses to cerebral ischemia/inflammation. Ann NY Acad Sci 823:132–147
Sanz M-J, Johnston B, Issekutz A, Kubes P (1999) Endothelin-1 causes P-selectin-dependent leukocyte rolling and adhesion within rat mesenteric microvessels. Am J Physiol 277 (Heart Circ Physiol 46):H1823–H1830
Vapaatalo H, Mervaala E (2001) Clinically important factors influencing endothelial function. Med Sci Monit 7:1075–1085
Oda M, Han J-Y, Nakamura M (2000) Endothelial cell dysfunction in microvasculature: relevance to disease processes. Clin Hemorheol Microcirc 23:199–211
Bertuglia S, Colantuoni A, Intaglietta M (1993) Effect of leukocyte adhesion and microvascular permeability on capillary perfusion during ischemia-reperfusion injury in hamster cheek pouch. Int J Microcirc Clin Exp 13:13–26
He P, Wang J, Zeng M (2000) Leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol 278:1686–1694
Michel CC, Curry FE (1999) Microvascular permeability. Physiol Rev 79:703–761
Muckart DJ, Bhagwanjee S (1997) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med 25:1789–1795
Lush CW, Kvietys PR (2000) Microvascular dysfunction in sepsis. Microcirculation 7:83–101
Karnik AM, Bashir R, Khan FA, Carvounis CP (1998) Renal involvement in the systemic inflammatory reaction syndrome. Renal Fail 20:103–116
Best WR, Becktel JM, Singleton JW, Kern F Jr (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70:439–444
Lankisch PG, Pohl U, Otto J, Rahlf G (1988) When should treatment of acute experimental pancreatitis be started? The early phase of bile-induced acute pancreatitis. Res Exp Med 188:123–129
Lienenlüke B, Stojanovic T, Fiebig T, Fayyazi A, Germann T, Hecker M (2001) Thalidomide impairment of trinitrobenzene sulphonic acid-induced colitis in the rat—role of endothelial cell-leukocyte interaction. Br J Pharmacol 133:1414–1423
Plusczyk T, Bersal B, Westermann S, Menger M, Feifel G (1999) ET-1 induces pancreatitis-like microvascular deterioration and acinar cell injury. J Surg Res 85:301–310
Plusczyk T, Bersal B, Menger M, Feifel G (2001) Differential effects of ET-1, ET-2, and ET-3 on pancreatic microcirculation, tissue integrity and inflammation. Dig Dis Sci 46:1343–1351
McCartney SA, Ballinger AB, Vojnovic I, Farthing MJ, Warner TD (2002) Endothelin in human inflammatory bowel disease: comparison to rat trinitrobenzenesulphonic acid-induced colitis. Life Sci 71:1893–1904
Naicker S, Bhoola KD (2001) Endothelins: vasoactive modulators of renal function in health and disease. Pharmacol Ther 90:61–88
Pollock DM (2001) Endothelin antagonists in the treatment of renal failure. Curr Opin Invest Drugs 2:513–520
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kruschewski, M., Anderson, T., Loddenkemper, C. et al. Endothelin-1 Receptor Antagonist (LU-135252) Improves the Microcirculation and Course of TNBS Colitis in Rats. Dig Dis Sci 51, 1461–1470 (2006). https://doi.org/10.1007/s10620-005-9019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9019-7